Genetic engineering and GMOs
Genetically modified organisms (GMOs) are organisms whose genetic make-up has been specifically altered using genetic engineering methods. Gene segments are transferred, e.g. to give plants certain characteristics that are very difficult or impossible to achieve with traditional breeding. Genetically modified plants are also referred to as GM events (derived from the transformation event during production) or GM lines (derived from the crossing of the plant's parent lines).
Novel genomic techniques (NGT) - also known as "new breeding techniques" - are genetic engineering techniques that have been used since 2001. They include genome editing techniques (e.g. CRISPR/Cas), which enable the targeted modification of genetic material. In the EU, plants produced by genome editing are subject to GMO legislation.
In Austria, the cultivation of genetically modified (GM) plants is prohibited. Worldwide, however, the area under cultivation is increasing every year. We therefore carry out monitoring of foodstuffs, animal feed and seeds. On behalf of the Federal Ministry of Labour, Social Affairs, Health, Care and Consumer Protection (BMASGPK) and the Federal Ministry of Agriculture, Forestry, Climate and Environmental Protection, Regions and Water Management (BMLUK), we also draw up expert opinions on seed, plant cultivation and analytical issues as well as on risks to health, the environment, animal nutrition, biodiversity and humans.
At national level, we provide technical advice to our two owner ministries and contribute the expertise of the GMO Task Force for parliamentary enquiries to the Codex Commission of the Austrian Food Codex (Codex Alimentarius Austriacus, ÖLMB). We participate in research programmes and publish scientific statements in the field of green genetic engineering (genetic engineering of plants).
At European level, our scientists contribute to improving the quality of applications for the authorisation of GM varieties. They check the quality of authorisation dossiers and contribute to the work of the European Food Safety Authority (EFSA) with their scientific risk assessments - and thus to food safety at European level.
Legal basis
- Regulation (EC) No. 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed.
- Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC
- Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning traceability and labelling of genetically modified organisms and traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC
- CommissionImplementing Regulation (EU) No. 503/2013 of 3 April 2013 concerning applications for authorisation of genetically modified food and feed pursuant to Regulation (EC) No. 1829/2003 of the European Parliament and of the Council and amending Commission Regulations (EC) No. 641/2004 and (EC) No. 1981/2006.
- Genetic Engineering Act (GTG): Austrian federal law regulating work with genetically modified organisms, the release and placing on the market of genetically modified organisms and the use of gene analysis and gene therapy on humans, and amending the Product Liability Act (BGBl. Nr. 510/1994)
- Ordinance of the Federal Minister of Agriculture, Forestry, Environment and Water Management on the Contamination of Seed with Genetically Modified Organisms and the Labelling of GMO Varieties and Seed of GMO Varieties (BGBl. II No. 478/2001)
Further links
Genetic engineering - Tasks of the Federal Ministry of Agriculture, Regions and Tourism
European Commission, Directorate General Health and Consumers - Technical Information on GMOs
European Food Safety Authority (EFSA) GMO Panel
Biosafety Clearing House - GMO Register
Federal Environment Agency - Technical Information on Genetic Engineering
Risks and dangers
The following risk areas arise for food safety in connection with GMO products:
- Toxic or immunological effects on consumers due to changes in the plant genome or triggered by transgenic proteins.
- Negative health effects for consumers due to nutritional changes in food/feed triggered by the genetic modification.
- Increase in herbicide residues in drinking water due to increased herbicide use in the cultivation of herbicide-resistant GMOs.
Ecological hazards may arise due to the following phenomena:
- Transfer of the genetic modification to conventional crops or to related wild plants or weeds can result in plant varieties with characteristics that were not intended and that have a negative impact on the affected ecosystem.
- Genetic modification of the plant can affect the ability of the seed to survive in the soil, which can exacerbate weed problems.
- Passing antibiotic resistance marker genes to bacteria can negatively affect the efficacy of antibiotics used in human and veterinary medicine.
- Genetic modification of the plant may have adverse effects on non-target (beneficial) organisms (e.g., toxic effects of transgenic proteins).
- The use of genetically modified plants can lead to the development of resistance in plant pests or weeds.
A particular economic and ecological challenge is the problem of coexistence between GMO cultivation and conventional/organic farming. This aspect concerns not only the cultivation of crops, but also the transport and further processing processes.
Risk assessment
Risk assessment addresses potential adverse effects on human and animal health or on the environment from genetically modified organisms and products derived from them.
The precise requirements for carrying out the risk assessment and for the data material to be submitted are governed by the following legal texts:
In addition to the specific legal regulations, genetically modified foods, like all other foods, are subject to the principles and requirements of European food law(Regulation (EC) No. 178/2002).
In the context of a GMO approval procedure for commercial purposes, applicants are in principle required to submit all data demonstrating that the GMO and any product produced from it will not have adverse effects on human and animal health or on the environment.
The scientific evaluation of the submitted data material and thus the risk assessment is carried out centrally by the European Food Safety Authority (EFSA). The EU Member States can also inspect the data and submit an opinion to EFSA within a three-month review period. Austria regularly contributes to the GMO risk assessment process by providing scientifically sound opinions.
The Austrian comments on GMO applications are prepared by our "TaskForce GMO". Its coordination is the responsibility of our department Integrative Risk Assessment Data and Statistics (DSR). The TaskForce GMO, which was founded in 2008, comprises more than 40 experts from the various business units and departments.
At the end of the assessment of a GMO application, an agreed Austrian statement is prepared in the DSR department, taking into account expert opinions from the Federal Environment Agency and possibly other external experts. The Austrian opinions are sent directly from there to EFSA, which collects all national opinions and incorporates them into its scientific assessment.
In addition to applications for authorisation, the TaskForce GMO deals with many other issues relating to GMOs and GMO risk assessment. These include, above all, statements on the scientific justification of Austrian import and cultivation bans, technical expert opinions and assessments on current topics, research projects, responses to inquiries, correspondence and scientific dialogue with external experts and EFSA, detection and control along the food and feed chain, participation in committees and technical conferences, assessment of future developments and data collection.
Approval
Food and feed produced from genetically modified organisms (GMOs) have been on the market in the European Union since 1996. The majority of these are animal feeds produced from GM plants and imported into the EU.
In order to be allowed to place GMO products on the market in the EU, they must undergo a special approval procedure. In this approval procedure, all data must be submitted by the party submitting the application to prove that the product has no negative effects on human and animal health or on the environment.
Approved Events & Varieties (EU)
Currently, food and feed derived from transgenic maize, soybean, oilseed rape, cotton and sugar beet are approved for marketing within the EU; the only GM event approved for cultivation in the EU is maize MON810 ("YieldGard"), for which cultivation is banned in Austria.
The GM products currently approved in the EU under Regulation (EC) No. 1829/2003 are listed in the European GMO Register.
If a farmer wants to use a genetically modified (GM) variety, both the event and the variety must be approved. Only when the event is approved can national authorities test varieties that have that event in the variety approval process.
The variety testing of a GM variety, as in the approval of traditionally bred varieties, comprises two parts:
- In the register test (Distinctness Uniformity Stability), numerous characteristics are collected, which are used to determine the distinctness, uniformity and stability of a variety.
- A "value for cultivation and use" is only assigned to a variety if the sum of its value-determining characteristics represents an improvement over the already approved varieties.
In Austria, due to legal requirements, no GM varieties are tested and therefore cannot be approved and cultivated. In individual EU member states, e.g. Spain, GM varieties are listed nationally. This allows these GM varieties to be included in the EU list (Common Catalogue of Varieties of Agricultural Plant Species). Listed GM varieties can only be cultivated in EU member states if there are no national bans in these countries as in Austria.
In the EU list, GM varieties are clearly identified by footnotes referring to the approved event.
Approval of GMOs as food and feed
The vast majority of GMO approval procedures concern their use as food and/or feed. The procedure and requirements for these approval procedures are regulated by Regulation (EC) No 1829/2003 as follows:
The application dossier for the authorization of a new GMO is submitted by the applicant to a national competent authority. In Austria, the competent national authority is the Federal Ministry of Labour, Social Affairs, Health, Care and Consumer Protection (BMASGPK). The application dossier contains an extensive collection of data on the genetic modification of the GMO, which should enable a scientific risk assessment with regard to possible adverse effects. If the application concerns products containing a genetically modified organism, an environmental risk assessment must also be carried out and a monitoring plan submitted.
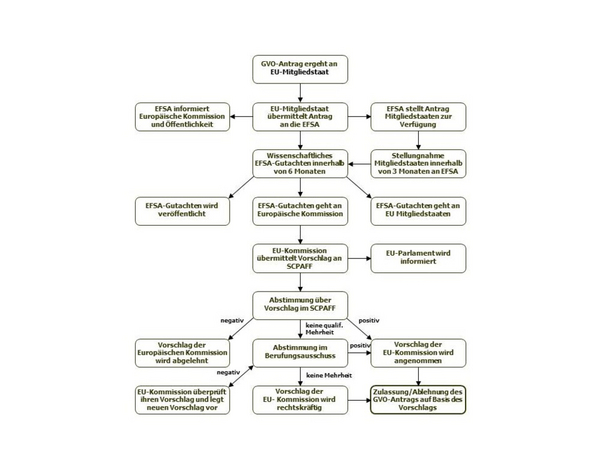
The national authority immediately forwards the application dossier to the European Food Safety Authority (EFSA), which reviews the dossier for completeness and makes it available to all EU member states. From this point, EFSA has six months to provide a scientific opinion on the dossier. Member states have three months to submit a technical assessment of the dossier to EFSA. The national assessments are then incorporated into the EFSA opinion. If additional data are requested by the EFSA GMO Panel, the six-month review period may be exceeded.
The EFSA opinion is forwarded to the European Commission and published. Within three months, the Commission makes a proposal to approve or reject the GMO, which is sent to the Standing Committee on the Food Chain and Animal Health(SCPAFF). This committee votes on the proposal. If it is approved by qualified majority, the Commission can implement the decision immediately. Without a qualified majority in SCPAFF, the draft decision is submitted to an Appeal Committee. A simple majority in the Appeals Committee is sufficient to reach a decision. If no majority can be reached in the Appeal Committee, the European Commission takes a decision. A GMO authorization is valid for a period of ten years.
Approval of GMOs for other purposes
The approval of GMOs for industrial or other purposes (e.g. wood production, cut flowers) is subject to the procedure under Directive 2001/18/EC, which regulates the release of the GMO into the environment:
The application dossier for the authorization of a new GMO is submitted to the national competent authority. This authority sends an assessment report to the applicant within 90 days and a summary of the dossier to the European Commission, which in turn sends it to the Member States within 30 days. The Member States raise their objections to an authorization within 30 days.
The national competent authority finally decides either for or against the approval of the GMO. Any objections by Member States to an authorization must be addressed by the applicant. Authorization is granted for a maximum period of ten years.
Questions and answers
Genetic engineering is the scientific discipline that deals with the targeted manipulation of genetic material in organisms. It is a subfield of biotechnology. The product created by the application of genetic engineering is a GMO.
GMO stands for Genetically Modified Organism (GMO). A GMO is an organism whose genetic material is deliberately modified in a way that does not occur in nature. GMOs therefore possess "transgenic characteristics", and are therefore also referred to as "transgenic organisms".
The majority of GMOs are genetically modified microorganisms, but plants or animals can also be the target of genetic modification.
GMOs can be purposefully engineered and adapted for specific processes and production steps. In contrast to natural or conventionally bred organisms, GMOs have characteristics that offer an advantage in many areas (e.g. industry, medicine, research). Genetically modified bacteria and fungi are used commercially for the most part, but genetically modified plants are also used to a lesser extent.
The majority of genetically modified plants on the market offer advantages in the field of agriculture because they are resistant to pests or herbicides.
Genetically modified animals are used primarily for experimental purposes. There are also already research approaches for releases - e.g. to control mosquito-borne infectious diseases such as malaria or dengue fever.
The main focus of our work is the assessment and clarification of possible negative effects of genetically modified organisms (GMOs) on animal and human health. Other important tasks include ensuring freedom from genetic engineering in the seed sector, dealing with issues relating to coexistence (of conventional/organic agriculture and GMO cultivation) and control in the food and feed sector.
To fulfill these tasks, we conduct risk assessment and safety research. We play a key role in the scientific evaluation of GMO approval documents as part of the EU approval process. Our experts engage in ongoing knowledge exchange with national and international experts and authorities, such as the "GMO Panel" of the European Food Safety Authority (EFSA). We draft statements and advise the Ministry of Health and the Ministry of Agriculture on issues related to genetic engineering. For the detection of GMOs we use state-of-the-art Real Time PCR analysis methods.
More information about our research projects can be found here.
A distinction is made between white, red, gray and green biotechnology or genetic engineering. White biotechnology relates to industrial applications: Use of biological processes to produce industrial products. Red bio technology concerns medical applications: For example, the diagnosis of a disease by means of gene analysis, somatic gene therapy or the production of drugs with the help of genetic engineering processes. Gray bio technology concerns environmental applications: Use of biological processes for soil remediation, waste disposal or wastewater treatment. Green biotechnology concerns applications in the field of plants: Utilization of plant ingredients for technological purposes or the use and utilization of genetically modified plants.
However, a clear demarcation between the individual areas is not always possible.
Furthermore, a distinction can be made between "contained use" and "release" applications of GMOs. Almost all applications with genetically modified microorganisms take place in the contained area. Here, safety measures are taken to ensure that no GMOs can enter the environment. Commercial applications in the field of green genetic engineering, on the other hand, are almost always releases, since plants are cultivated in fields and plant material is used as a raw material for industrial products (e.g. animal feed, food).
By definition, the "release" of GMOs is the deliberate introduction of GMOs into the environment for development and experimental purposes, e.g. field tests with genetically modified plants for research purposes. "Placing on the market" is also a form of release. By definition, it means making GMOs available as or in products to third parties. What is meant by this is the sale or transfer of products containing or consisting of GMOs. Such products can be food and feed, but also seeds or other (non-replicable) plant material.
The main goals pursued under green genetic engineering:
- Tolerance to herbicides
- Resistance to pests (e.g. insects)
- Resistance to diseases (e.g. fungi, viruses)
- Stress tolerance (cold, drought, salt tolerance)
- Enrichment of ingredients (e.g. fatty acids, vitamins, minerals)
- Modification of ingredients (e.g. amylose-reduced starch, lignin-reduced wood)
- Elimination of undesirable substances (e.g. alkaloids)
- Production of industrial raw materials (e.g. bioplastics)
- Longer shelf life due to delayed ripening
- Bioremediation of soils
- Change of flower color
Genetically modified plants currently approved in the EU generally possess one or more of the traits listed below:
- Herbicide tolerance (insensitivity to certain herbicides).
- Insect pest resistance (a toxin is effective against certain plant pests)
- pollen sterility (to simplify plant breeding)
- improved industrial utilization (brings advantages for further processing)
- altered flower color (ornamental purposes)
- marker genes (used to select transformed plant cells during development of the GMO in the laboratory)
- improved nutritional value
Currently, research laboratories are working on genetically modified plants with the following characteristics:
- Virus, fungus, nematode, bacterial resistance.
- tolerance to drought, cold, salinity and nutrient deficiency
- delayed maturity
- elimination of undesirable ingredients
- production of therapeutically active substances
If several transgenic traits are used or "stacked" in genetically modified plants, this is referred to as "stacked events". As a rule, this is done by conventional crossing of two genetically modified plants. For the approval of a stacked event, similar criteria apply as for "single events". Before a stacked event is approved, the risk assessment of each single event involved must be completed. The main focus of the risk assessment of the stacked event is the estimation of possible negative effects caused by the interaction of the individual transgenic traits.
The GMO approval process is regulated uniformly for all 27 EU member states: Applications for approval are subject to a risk assessment by the European Food Safety Authority (EFSA). Member states can inspect all dossiers and submit an opinion as part of this review. Based on the results of the risk assessment by EFSA and the comments of the Member States, the European Commission then formulates a proposal for the decision to authorize or not to authorize. This decision is discussed and voted on in the Standing Committee. If no consensus is reached, the European Commission makes the final decision.
Foods that contain approved GMOs, are produced from approved GMOs or contain ingredients produced from approved GMOs must be labeled as "genetically modified". Foods with a GMO content of less than 0.9% are not required to be labeled if these traces are adventitious or technically unavoidable. Labeling occurs regardless of whether DNA or protein can be detected in the final product due to the genetic modification. In the case of GMOs not approved in the EU, "zero tolerance" applies, i.e. if an unapproved GMO is detected, the supplies or products in question are generally not marketable. The only exception is GMOs in feed that have already been risk assessed, for which a tolerance of 0.1% applies. Labelling is also mandatory for GM feed and seed of GM varieties.
Contact
DI Dr. Alexandra Ribarits
- anfragen@ages.at
-
Spargelfeldstraße 191
1220 Wien
Dr. Markus Wögerbauer
- anfragen@ages.at
-
Spargelfeldstraße 191
1220 Wien
Last updated: 02.04.2025
automatically translated