In order to better understand the relationship between antibiotic resistance and use, good quality data is essential. Manufacturers, marketing authorisation holders (depositors) and pharmaceutical wholesalers must report the distribution of veterinary medicinal products containing antimicrobial substances. In addition, veterinarians running in-house pharmacies report the quantities of antibiotics dispensed to farms. The legal basis for the collection of this data is the Veterinary Antibiotics Volume Flow Ordinance.
This report presents the distribution and dispensing quantities of antibiotics authorised in veterinary medicine for farm animals in 2023 and compares them with previous years. The total distribution volume of antimicrobial substances for livestock has decreased by 5% compared to the previous year and amounts to 32.54 tonnes in 2023. The sales volume of antibiotics classified by the WHO as "antibiotics of the highest importance for human medicine" has decreased by 8% since the previous year, from 4.35 to 4 tonnes. Over the years, these groups of active ingredients have accounted for a relatively constant share of around 12% of the total quantity.
In general, sales volumes have fluctuated in recent years. These can be attributed on the one hand to the availability of some veterinary medicinal products and on the other hand to the build-up and reduction of stocks. The underlying animal population has changed only slightly over the last few years, which is why the changes in the distribution volume cannot be explained by lower or higher animal numbers.
As part of their dispensing report, veterinarians who run in-house pharmacies state the animal species for which the antibiotic was dispensed. This allows the dispensing quantities of antimicrobially active substances to be allocated to the animal species. In order to be able to compare the dispensing quantities of the different animal species with each other, these must be standardised on the basis of the respective animal population. For this purpose, the European Medicines Agency (EMA) has introduced the standardisation factor PCU. This is a technical measure and relates to one kilogramme of body weight. This results in standardised values for pigs of 54.5 mg/PCU (+0.6 mg/PCU compared to the previous year), for cattle of 16.4 mg/PCU (-0.6 mg/PCU compared to the previous year) and for poultry of 19.5 mg/PCU (+0.8 mg/PCU). These figures are still subject to greater uncertainty, as no directly applied antibiotics are recorded as part of the mandatory dispensing quantity survey.
Introduction
In Austria, the Veterinary Antibiotics Volume Flow Regulation (Federal Law Gazette II No. 83/2014, last amended by Federal Law Gazette II No. 127/2022) provides the legal basis for a system to record the distribution and consumption of antibiotics in the veterinary sector. According to §4(2), AGES has the task of compiling a report on the evaluation of the data and publishing it via the Federal Ministry of Social Affairs, Health, Care and Consumer Protection.
This report presents the distribution and dispensing quantities of antibiotics that were authorised in veterinary medicine for farm animals in the years 2019 to 2023.
In addition to the national regulation, the new veterinary medicinal products legislation (Regulation (EU) 2019/6) has been in force in the EU since 28 January 2022. In combination with Delegated Regulations (EU) 2021/578 and (EU) 2021/1248, which were adopted on the basis of Article 57, it sets out the requirements for collecting data on antimicrobial medicinal products used in veterinary medicine.
Sales volumes
Since 2014, the data on the distribution of veterinary medicinal products containing antibiotics has been reported electronically by manufacturers, marketing authorisation holders (distributors) and medicinal product wholesalers to the AGES Medical Market Surveillance database(further information on reporting data). The reported data includes the marketing authorisation number and the number of packages sold per veterinarian in charge of the in-house pharmacy.
The total quantity of active substance sold in tonnes is calculated from this data.
Dispensing and application quantities
In addition, since 2016, veterinarians in charge of in-house pharmacies have had to upload their data on the dispensing of antibiotics for use on livestock (except horses, see Veterinary Antibiotics Volume Flow Regulation §7(2)) for the previous year either independently or via recognised reporting points electronically in the AGES eService in accordance with the Veterinary Antibiotics Volume Flow Regulation. Data on the veterinary medicinal products used directly on animals (application data) are also required for a complete presentation. These can be reported for the first time for 2023 in the same way as the dispensing data. This data also includes information on the type of animal and use. This makes it possible to allocate the quantity of antimicrobially active substances to individual animal species. For reasons of comparability, however, only the quantities released are being analysed at this stage. The applications reported in the current year 2023 are shown in the Application data section.
ATCvet categories
The ATCvet system (World Health Organization Collaborating Centre for Drug Statistics Methodology, n.d.) is used to classify the active substances in analogy to the anatomical-therapeutic-chemical (ATC) classification system used in human medicine. The veterinary medicinal products whose distribution and dispensing data must be reported are listed in the following table. Antiparasitics with the ATCvet code "QP51AG" were not authorised in Austria during the evaluation period and therefore do not appear in the analyses.
Conversion factors were provided by the EMA for those antibiotics that are specified in international units (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption 2022).
| Category | ATCvet code |
|---|---|
| AB for intestinal use | QA07AA; QA07AB |
| AB for intrauterine use | QG01AA; QG01AE; QG01BA; QG01BE; QG51AA; QG51AG |
| AB for systemic use | QJ01 |
| AB for intramammary use | QJ51 |
| Antiparasitics | QP51AG |
Categories considered and associated ATCvet codes
The analyses were created using the programming language R (R Core Team 2024).
Results of the sales volume survey
Compared to 2022, the total quantity sold in 2023 decreased by 1.72 tonnes. This corresponds to a relative decrease of 5 %.
| Year | Sales volume | Difference (absolute) | Difference (relative) |
|---|---|---|---|
| 2019 | 40,51 | - | - |
| 2020 | 43,65 | 3,14 | 7,7 % |
| 2021 | 39,07 | -4,58 | -10,5 % |
| 2022 | 34,26 | -4,81 | -12,3 % |
| 2023 | 32,54 | -1,72 | -5,0 % |
Looking at the type of application, oral preparations (powders, tablets and pastes) for the treatment of individual animals or groups of animals are far ahead of the other forms of application in 2023 at 25.82 tonnes (79.4% of the total sales volume). Preparations for parenteral use are in second place with 5.02 tonnes (15.4 %), followed by the group of intramammary applications (0.95 tonnes or 2.9 %), to which dry feeders were also allocated. The premixes of medicated feed used orally for herd treatment account for 0.63 tonnes (1.9 %).
In terms of sales volumes broken down by active ingredient group , tetracyclines remain in first place with 15.3 tonnes (47.1 % of total sales volumes), followed by extended-spectrum penicillins with 5.1 tonnes (15.7 %), sulphonamides with 3.3 tonnes (10.2 %) and macrolides with 2.3 tonnes (7.1 %). The largest decreases were recorded for extended-spectrum penicillins (-0.8 tonnes) and tetracyclines (-0.5 tonnes). In contrast, there were slight increases in sulphonamides (+0.08 tonnes), trimethoprim and derivatives (+0.02 tonnes), fluoroquinolones (+0.04 tonnes), pleuromutilins (+0.06 tonnes) and lincosamides (+0.06 tonnes). The classification of active substances into active substance groups was carried out in accordance with the EMA guidelines (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption 2022). The "other antibiotics" group includes "rifaximin" and "spectinomycin".
Analyses of the active ingredient groups of macrolides, fluoroquinolones, 3rd and 4th generation cephalosporins and polymyxins, which are classified by the WHO as antibiotics of very high importance for human medicine due to their status, are also presented separately in the section Antibiotics of very high importance for human medicine.
Quantities sold by active ingredient group for the years 2019 to 2023 and the difference between 2023 and 2022 in tonnes
| Active ingredient group | 2019 | 2020 | 2021 | 2022 | 2023 | Diff. |
|---|---|---|---|---|---|---|
| Tetracyclines | 19,72 | 22,11 | 19,30 | 15,78 | 15,27 | -0,50 |
| Penicillins with extended spectrum | 6,60 | 7,35 | 6,26 | 5,94 | 5,13 | -0,81 |
| Sulfonamides | 3,92 | 3,46 | 3,66 | 3,18 | 3,26 | 0,08 |
| Macrolides | 2,97 | 3,47 | 2,48 | 2,60 | 2,33 | -0,27 |
| Beta-lactamase sensitive penicillins | 1,56 | 1,58 | 1,64 | 1,69 | 1,50 | -0,18 |
| Aminoglycosides | 1,28 | 1,29 | 1,32 | 1,34 | 1,32 | -0,02 |
| Polymyxine | 1,53 | 1,54 | 1,47 | 1,11 | 1,02 | -0,09 |
| Trimethoprim and derivatives | 0,78 | 0,69 | 0,73 | 0,64 | 0,65 | 0,02 |
| Fluoroquinolones | 0,46 | 0,48 | 0,46 | 0,41 | 0,45 | 0,04 |
| Beta-lactamase resistant penicillins | 0,41 | 0,38 | 0,41 | 0,42 | 0,43 | <0,01 |
| Amphenicole | 0,47 | 0,42 | 0,39 | 0,39 | 0,36 | -0,03 |
| Pleuromutiline | 0,39 | 0,41 | 0,33 | 0,26 | 0,32 | 0,06 |
| 3rd +4th generation cephalosporins | 0,22 | 0,23 | 0,23 | 0,22 | 0,20 | -0,03 |
| Lincosamides | 0,10 | 0,09 | 0,20 | 0,11 | 0,17 | 0,06 |
| Other antibiotics | 0,07 | 0,09 | 0,16 | 0,13 | 0,08 | -0,04 |
| 1st + 2nd generation cephalosporins | 0,03 | 0,04 | 0,05 | 0,05 | 0,04 | <0,01 |
| Total | 40,51 | 43,65 | 39,07 | 34,26 | 32,54 | -1,72 |
Antibiotics for intestinal use
Compared to 2022, there was a decrease in the quantities of antibiotics for intestinal use (ATCvet QA07) sold in 2023. See table below for details.
Quantities sold by active substance group (for intestinal use) for the years 2019 to 2023 and the difference between 2023 and 2022 in tonnes
| Active ingredient group | 2019 | 2020 | 2021 | 2022 | 2023 | Diff. |
|---|---|---|---|---|---|---|
| Polymyxine | 1,53 | 1,54 | 1,47 | 1,11 | 1,02 | -0,09 |
| Aminoglycosides | 0,23 | 0,27 | 0,32 | 0,39 | 0,45 | 0,06 |
| total | 1,76 | 1,81 | 1,79 | 1,50 | 1,47 | -0,03 |
Antibiotics for intramammary use
The following table shows the quantities of antibiotics administered intramammarily (ATCvet QJ51), broken down into dry feed and other preparations used during lactation. The quantities have decreased slightly compared to the previous year.
Quantities sold by active ingredient group (for intramammary use) for the years 2019 to 2023 and the difference between 2023 and 2022 in tonnes
| Active ingredient group | 2019 | 2020 | 2021 | 2022 | 2023 | Diff. |
|---|---|---|---|---|---|---|
| Beta-lactamase sensitive penicillins | 0,32 | 0,35 | 0,36 | 0,34 | 0,31 | -0,03 |
| 1st + 2nd generation cephalosporins | 0,02 | 0,03 | 0,04 | 0,04 | 0,03 | -0,01 |
| Aminoglycosides | 0,01 | 0,02 | 0,02 | 0,02 | 0,02 | 0,00 |
| 3rd +4th generation cephalosporins | 0,03 | 0,03 | 0,02 | 0,03 | 0,02 | -0,01 |
| Lincosamides | 0,02 | 0,01 | 0,02 | 0,02 | 0,01 | -0,01 |
| Penicillins with extended spectrum | 0,01 | 0,01 | 0,01 | 0,01 | 0,01 | 0,00 |
| Subtotal 'During lactation' | 0,42 | 0,45 | 0,48 | 0,46 | 0,40 | -0,06 |
| Beta-lactamase resistant penicillins | 0,37 | 0,37 | 0,38 | 0,41 | 0,41 | 0,00 |
| Beta-lactamase sensitive penicillins | 0,08 | 0,08 | 0,07 | 0,07 | 0,05 | -0,02 |
| Penicillins with extended spectrum | 0,02 | 0,00 | 0,02 | 0,03 | 0,04 | 0,01 |
| Aminoglycosides | 0,04 | 0,04 | 0,03 | 0,03 | 0,02 | -0,02 |
| 1st + 2nd generation cephalosporins | 0,01 | 0,01 | 0,01 | 0,01 | 0,01 | 0,00 |
| Other antibiotics | <0,01 | 0,01 | 0,01 | 0,01 | 0,01 | 0,00 |
| 3rd +4th generation cephalosporins | 0,01 | 0,01 | 0,01 | 0,01 | 0,01 | 0,00 |
| Subtotal 'dry setter' | 0,53 | 0,51 | 0,54 | 0,58 | 0,55 | -0,03 |
| total | 0,94 | 0,96 | 1,02 | 1,04 | 0,95 | -0,08 |
Antibiotics for systemic use
The following two tables show the sales volumes of antibiotics for systemic use (ATCvet QJ01) by active ingredient group and form of application. The largest decreases were recorded for penicillins (-0.82 tonnes) and tetracyclines (-0.48 tonnes). With regard to the form of application, a larger decrease was recorded for oral antibiotics (-1.38 tonnes).
Quantities sold by active ingredient group (for systemic use) for the years 2019 to 2023 and the difference between 2023 and 2022 in tonnes
| Active ingredient group | 2019 | 2020 | 2021 | 2022 | 2023 | Diff. |
|---|---|---|---|---|---|---|
| Tetracyclines | 19,67 | 22,05 | 19,21 | 15,68 | 15,20 | -0,48 |
| Penicillins with extended spectrum | 6,53 | 7,32 | 6,20 | 5,88 | 5,06 | -0,82 |
| Sulfonamides | 3,92 | 3,46 | 3,66 | 3,18 | 3,26 | 0,08 |
| Macrolides | 2,97 | 3,47 | 2,48 | 2,60 | 2,33 | -0,27 |
| Beta-lactamase sensitive penicillins | 1,16 | 1,15 | 1,21 | 1,27 | 1,14 | -0,13 |
| Aminoglycosides | 1,00 | 0,96 | 0,94 | 0,91 | 0,83 | -0,08 |
| Trimethoprim and derivatives | 0,78 | 0,69 | 0,73 | 0,64 | 0,65 | 0,01 |
| Fluoroquinolones | 0,46 | 0,48 | 0,46 | 0,41 | 0,45 | 0,04 |
| Amphenicole | 0,47 | 0,42 | 0,39 | 0,39 | 0,36 | -0,03 |
| Pleuromutiline | 0,39 | 0,41 | 0,33 | 0,26 | 0,32 | 0,06 |
| 3rd +4th generation cephalosporins | 0,18 | 0,19 | 0,19 | 0,19 | 0,17 | -0,02 |
| Lincosamides | 0,08 | 0,08 | 0,18 | 0,09 | 0,15 | 0,06 |
| Other antibiotics | 0,07 | 0,08 | 0,15 | 0,11 | 0,07 | -0,04 |
| Total | 37,67 | 40,77 | 36,13 | 31,60 | 29,99 | -1,61 |
Quantities sold by form of application (for systemic use) for the years 2019 to 2023 and the difference between 2023 and 2022 in tonnes
| Form of application | 2019 | 2020 | 2021 | 2022 | 2023 | Diff. |
|---|---|---|---|---|---|---|
| Oral | 30,95 | 34,13 | 29,89 | 25,72 | 24,34 | -1,38 |
| Parenteral | 5,49 | 5,50 | 5,38 | 5,25 | 5,02 | -0,23 |
| Premix | 1,23 | 1,14 | 0,87 | 0,63 | 0,63 | 0,00 |
| Total | 37,67 | 40,77 | 36,13 | 31,60 | 29,99 | -1,61 |
Antibiotics for intrauterine use
The sales volumes of antibiotics for intrauterine use (ATCvet QG01, QG51) are shown in the following table for each active ingredient group. Only tetracyclines showed a slight decrease compared to the previous year.
Quantities sold by active ingredient group (for intrauterine use) for the years 2019 to 2023 and the difference between 2023 and 2022 in tonnes
| Active ingredient group | 2019 | 2020 | 2021 | 2022 | 2023 | Diff. |
|---|---|---|---|---|---|---|
| Tetracyclines | 0,06 | 0,06 | 0,08 | 0,10 | 0,07 | -0,03 |
| Beta-lactamase resistant penicillins | 0,04 | 0,02 | 0,02 | 0,01 | 0,02 | 0,01 |
| Penicillins with extended spectrum | 0,04 | 0,02 | 0,02 | 0,01 | 0,02 | 0,01 |
| total | 0,14 | 0,10 | 0,13 | 0,12 | 0,11 | -0,01 |
Antibiotics of paramount importance for human medicine
The active substance groups macrolides, fluoroquinolones, 3rd and 4th generation cephalosporins and also the group of polymyxins (including colistin) are classified by the WHO as so-called Highest Priority Critically Important Antimicrobials (HPCIA) due to their status (WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance and World Health Organisation 2017). The WHO also classifies 5th generation cephalosporins, ketolides and glycopeptides as HPCIAs; however, these do not have any sales volumes. The quantities of these active substance groups sold between 2019 and 2023 are shown in the following table and figure. In 2023, there was a decrease in macrolides in particular, while a slight increase was recorded for fluoroquinolones compared to the previous year.
Quantities of antibiotics of the highest importance for human medicine (HPCIA) sold for the years 2019 to 2023 and the difference between 2023 and 2022 in tonnes.
| Active substance group | 2019 | 2020 | 2021 | 2022 | 2023 | Diff. |
|---|---|---|---|---|---|---|
| Macrolides | 2,97 | 3,47 | 2,48 | 2,60 | 2,33 | -0,27 |
| Polymyxine | 1,53 | 1,54 | 1,47 | 1,11 | 1,02 | -0,09 |
| Fluoroquinolones | 0,46 | 0,48 | 0,46 | 0,41 | 0,45 | 0,04 |
| 3rd +4th generation cephalosporins | 0,22 | 0,23 | 0,23 | 0,22 | 0,20 | -0,02 |
| total | 5,17 | 5,72 | 4,64 | 4,35 | 4,00 | -0,35 |
In addition to the above-mentioned classification by the WHO, the EMA working group AMEG (Antimicrobial Advice Ad Hoc Expert Group) has carried out a new categorisation of antimicrobial active substances - based on possible consequences for public health - into four groups (European Medicines Agency, Committee for Medicinal Products for Veterinary use (CVMP), Committee for Medicinal Products for Human Use (CHMP) 2019):
- Category A: Avoid - do not use in food-producing animals
- Category B: Restrict - critically important for human medicine
- Category C: Caution - only to be considered if no clinically effective antibiotics from Category D are available
- Category D: Care - if possible as first-line therapy; only if necessary
The results of the sales volumes can be seen in the following figure, whereby group A does not occur in Austria.
Standardised sales quantities
In the previous chapters, only the distribution volumes recorded in recent years were compared with each other. No standardisation was carried out on the basis of the respective animals kept (animal populations per year). In order to take into account the different animal demographics of the countries and thus enable comparability, the Population Correction Unit (PCU) was defined as part of the EMA project "European Surveillance of Veterinary Antimicrobial Consumption (ESVAC)", which is calculated from stock and slaughter data as well as imports and exports. Further information on the calculation of the PCU can be found in Annex 3 of the report "Trends in the sales of veterinary antimicrobial agents in nine European countries: 2005-2009" (European Medicines Agency 2011).
The unit mg/PCU is a technical parameter for comparing quantities of different animal species, countries or years; 1 PCU = 1 kg.
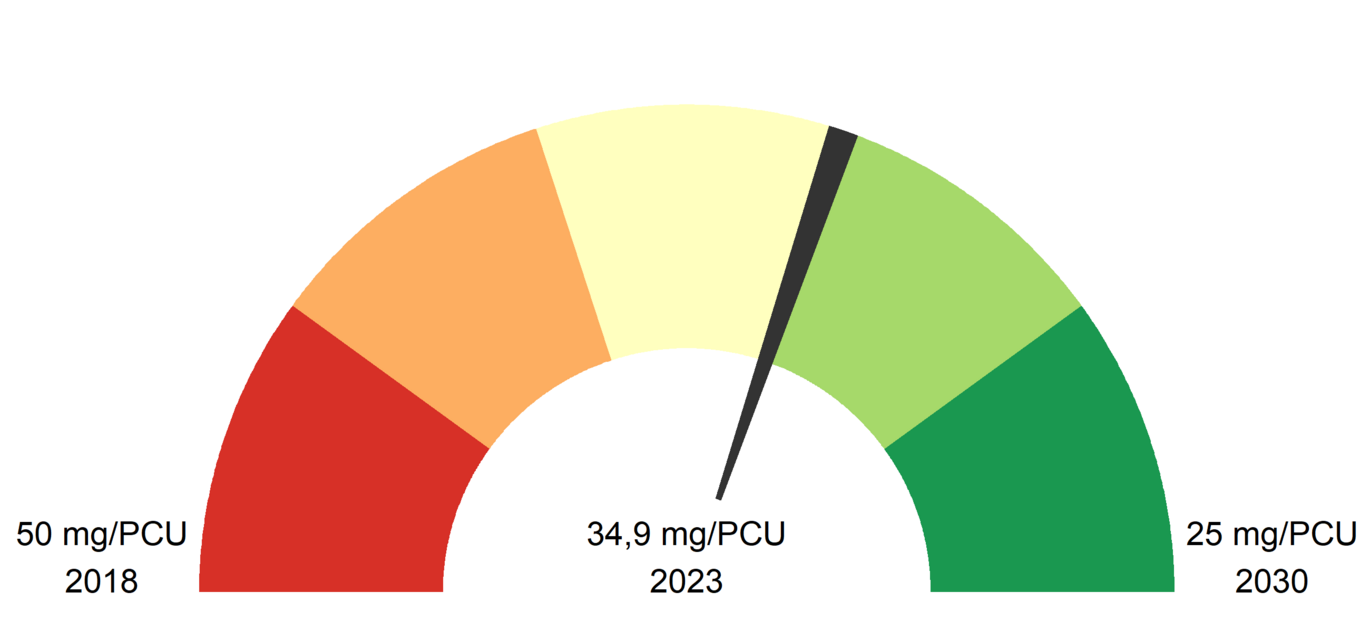
This standardisation factor shows only slight fluctuations for Austria over the last few years. This means that the changes in the quantities cannot generally be explained by higher or lower animal numbers in the respective years. The following figure shows the standardised distribution volumes based on the PCU. A reduction of -1.3 mg/PCU (-3.6 % compared to the previous year) can be seen in 2023.
The European Union's Farm to Fork Strategy (European Union 2020) sets the target of reducing the distribution volumes of antibiotics authorised for food-producing livestock by 50% by 2030 compared to 2019. The current progress for Austria can be seen in the following figure.
Sales volumes in the pet sector
The distribution volumes presented so far only include those veterinary medicinal products that are authorised for at least one type of livestock (or horses). However, all veterinary medicinal products with antimicrobial active substances are reported in the sales volume report, including those that are only authorised for pets (dogs, cats, etc.). The quantities of recent years are listed in the following table and range between 0.51 and 0.61 tonnes in 2023
| Year | Distribution volume | Difference (absolute) | Difference (relative) |
|---|---|---|---|
| 2019 | 0,59 | - | - |
| 2020 | 0,51 | -0,08 | -13,39 % |
| 2021 | 0,61 | 0,10 | 18,78 % |
| 2022 | 0,55 | -0,06 | -9,07 % |
| 2023 | 0,57 | 0,02 | 3,34 % |
Antimicrobial veterinary medicinal products imported into Austria from other Member States
Sales volumes of veterinary medicinal products with antimicrobial active substances brought to Austria were not taken into account in the previous reports, as these are not veterinary medicinal products authorised in Austria. However, veterinary medicinal products that are authorised in other EU/EEA countries can be obtained by veterinarians running in-house pharmacies in the event of a therapeutic emergency in accordance with the requirements of the Veterinary Medicinal Products Act (§§ 57-59) and the Medicinal Products Import Act (AWEG) and must be reported accordingly(further information on the AWE).
When reporting data to the EMA, the quantities of antimicrobial medicinal products obtained from other EU/EEA countries must be taken into account (Article 6 of Delegated Regulation (EU) 2021/578).
The quantity of active substances of these antimicrobial medicinal products shipped to Austria in 2023 amounted to 1.43 tonnes and thus accounted for around 4.1% of the total quantity. Antibiotics shipped to Austria are primarily preparations for oral use. Each species (cattle, pigs and poultry) accounted for approx. 400 - 500 kg of active ingredients. In poultry, this accounted for around 20% of the total antibiotics used in 2023.
Results of the survey of dispensing quantities
In 2023, 1774 veterinary in-house pharmacies (HAPO) were registered in Austria, of which 1579 also purchased antibiotics in 2023, with 95 % of antibiotics being sold to 345 HAPO (around 20 %). Conversely, this means that 80 % of HAPOs only purchase 5 % of the total quantity.
In the dispensing report, veterinarians in charge of in-house pharmacies must state which antibiotics were dispensed to which farms and in what quantities. A total of 565 out of 1774 HAPOs complied with this reporting obligation for the reporting year 2023.
In order to be able to check the completeness of the dispensing data, veterinarians running in-house pharmacies who are not obliged to submit a dispensing report in accordance with Veterinary Antibiotics Volume Flow Regulation §7(2) must submit a so-called empty dispensing report (see Veterinary Antibiotics Volume Flow Regulation §7(3)). This year, 1026 HAPOs carried out such a report. Of the 345 HAPOs mentioned above (top 95%), 325 submitted a dispensing notification or dispensing empty notification in 2023.
A total of around 26.7 tonnes of antibiotics dispensed to agricultural businesses were reported in 2023. The difference of around 5.8 tonnes (18%) compared to the reports submitted by manufacturers, depositors and pharmaceutical wholesalers can have various causes, such as use by veterinarians, dispensing to animal species not subject to mandatory reporting, stockpiling or non-reporting. The following table shows the quantities dispensed and distributed, as well as the respective differences and proportions.
| Notification year | Dispensing quantity | Distribution quantity | Difference: absolute | relative |
|---|---|---|---|---|
| 2019 | 33,17 | 40,51 | 7,34 | 18,11 % |
| 2020 | 33,51 | 43,65 | 10,14 | 23,23 % |
| 2021 | 30,86 | 39,07 | 8,21 | 21,02 % |
| 2022 | 27,70 | 34,26 | 6,56 | 19,14 % |
| 2023 | 26,67 | 32,54 | 5,86 | 18,02 % |
Species-related analyses
In addition to specifying which farms antibiotics were dispensed to, veterinarians who run in-house pharmacies must also report the animal species and type of use for which the antibiotics were dispensed. The following figure shows that 66% of the quantity was dispensed for pigs in 2023, followed by cattle with 26% and poultry with 7%.
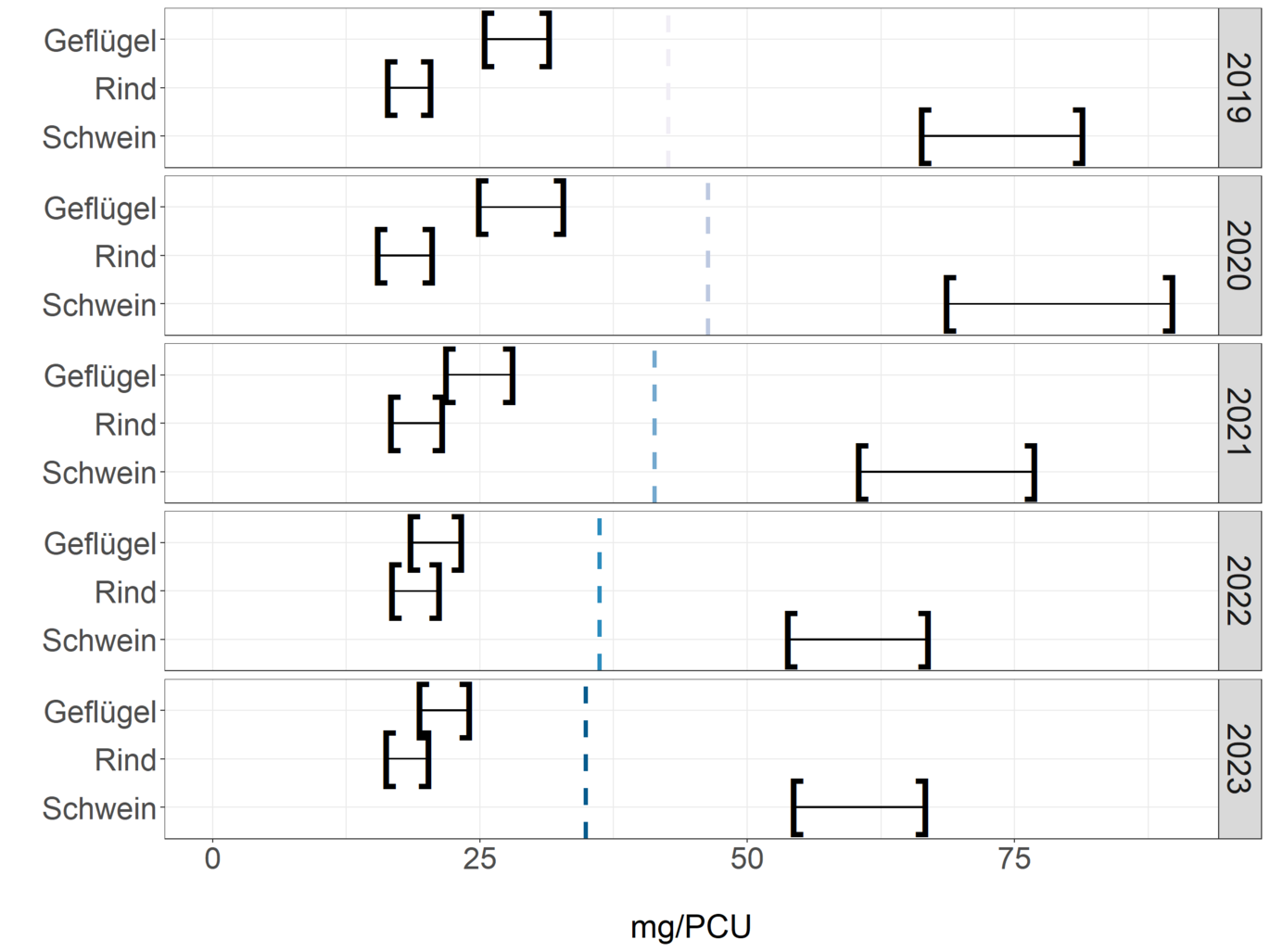
As the herds and slaughter numbers of different animal species differ, the release quantities for pigs, cattle and poultry are standardised in mg/PCU, as also shown in the ESVAC report (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption 2022) in the following table and figure. The left-hand bracket shows the standardised reported dispensing quantity. The total reported dispensing quantity is 18 % lower than the total distribution quantity. This difference was taken into account for the respective animal species and extrapolated in the right-hand brackets in the figure. The values shown in the graph can be seen in the table. These key figures are currently subject to greater uncertainty, as the use of AB in horses and pets is not taken into account here and the proportion of dispensing for use in cattle, pigs and poultry is not identical.
Standardised dispensing quantities based on PCU per animal species pig, cattle and poultry for the years 2019 to 2023. Column mg/PCU corresponds to the standardised reported dispensing quantities; column mg/PCU (extrapolated) shows the values extrapolated taking into account the reporting difference to the distribution quantity.
| Year | Animal species | mg/PCU | mg/PCU (extrapolated) |
|---|---|---|---|
| 2019 | Poultry | 25,6 | 31,3 |
| 2019 | Beef | 16,5 | 20,2 |
| 2019 | Pork | 66,5 | 81,2 |
| 2020 | Poultry | 25,1 | 32,6 |
| 2020 | Cattle | 15,6 | 20,4 |
| 2020 | Pork | 68,8 | 89,6 |
| 2021 | Poultry | 22,0 | 27,9 |
| 2021 | Cattle | 16,8 | 21,3 |
| 2021 | Pork | 60,6 | 76,7 |
| 2022 | Poultry | 18,7 | 23,1 |
| 2022 | Cattle | 17,0 | 21,0 |
| 2022 | Pork | 53,9 | 66,7 |
| 2023 | Poultry | 19,5 | 23,8 |
| 2023 | Cattle | 16,4 | 20,0 |
| 2023 | Pigs | 54,5 | 66,5 |
Quantities sold for pigs
The table shows the reported release quantities for pigs per active substance group in tonnes. A breakdown of the quantities dispensed for pigs by type of use is shown in the table below. This means, for example, that 25.8% of all antibiotics dispensed in 2023 were used in pig fattening.
Quantities dispensed for the animal species pig per active substance group in tonnes for the years 2019 to 2023
| Active ingredient group | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|
| Tetracyclines | 13,43 | 14,64 | 12,65 | 9,99 | 9,95 |
| Penicillins with extended spectrum | 4,41 | 4,37 | 4,22 | 4,29 | 3,99 |
| Macrolides | 1,82 | 1,69 | 1,50 | 1,44 | 1,26 |
| Sulfonamides | 1,64 | 1,31 | 1,14 | 0,95 | 0,90 |
| Polymyxine | 0,87 | 1,03 | 0,87 | 0,63 | 0,47 |
| Aminoglycosides | 0,52 | 0,47 | 0,27 | 0,25 | 0,22 |
| Pleuromutiline | 0,27 | 0,27 | 0,23 | 0,20 | 0,19 |
| Trimethoprim and derivatives | 0,33 | 0,26 | 0,23 | 0,19 | 0,18 |
| Beta-lactamase sensitive penicillins | 0,20 | 0,21 | 0,21 | 0,20 | 0,17 |
| Fluoroquinolones | 0,10 | 0,11 | 0,11 | 0,10 | 0,10 |
| Lincosamides | 0,04 | 0,05 | 0,09 | 0,06 | 0,10 |
| Amphenicole | 0,07 | 0,08 | 0,07 | 0,07 | 0,05 |
| Other antibiotics | 0,05 | 0,05 | 0,08 | 0,05 | 0,05 |
| 3rd +4th generation cephalosporins | 0,05 | 0,05 | 0,05 | 0,04 | 0,04 |
| Beta-lactamase resistant penicillins | <0,01 | <0,01 | <0,01 | <0,01 | <0,01 |
| 1st + 2nd generation cephalosporins | <0,01 | <0,01 | <0,01 | <0,01 | <0,01 |
| Total | 23,81 | 24,58 | 21,71 | 18,47 | 17,67 |
Percentage of sales volumes in relation to the total sales volume for the pig species per type of utilisation for the years 2019 to 2023
| Type of utilisation | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|
| Other | 8,8 % | 8,7 % | 7,4 % | 7,2 % | 6,6 % |
| Piglet rearing | 12,3 % | 10,1 % | 11,2 % | 11,1 % | 10,8 % |
| Mast | 29,5 % | 31,1% | 30,0 % | 26,5 % | 25,8 % |
| Breeding | 21,3 % | 23,5% | 21,7 % | 21,8 % | 23,1 % |
| Total | 71,8 % | 73,3% | 70,4 % | 66,7 % | 66,3 % |
Delivery quantities for cattle
The table shows the reported release quantities for cattle per active substance group in tonnes and the further table shows them as a percentage according to the reported type of use.
Release quantities for the animal species cattle per active substance group in tonnes for the years 2019 to 2023
| Active substance group | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|
| Tetracyclines | 3,28 | 3,36 | 3,80 | 3,83 | 3,54 |
| Beta-lactamase sensitive penicillins | 0,59 | 0,82 | 0,83 | 0,84 | 0,90 |
| Sulfonamides | 1,56 | 0,71 | 0,73 | 0,62 | 0,65 |
| Aminoglycosides | 0,38 | 0,49 | 0,47 | 0,55 | 0,60 |
| Beta-lactamase resistant penicillins | 0,26 | 0,27 | 0,29 | 0,40 | 0,34 |
| Penicillins with extended spectrum | 0,31 | 0,31 | 0,31 | 0,46 | 0,33 |
| Amphenicole | 0,17 | 0,15 | 0,15 | 0,17 | 0,18 |
| Trimethoprim and derivatives | 0,31 | 0,14 | 0,15 | 0,12 | 0,13 |
| Macrolides | 0,09 | 0,09 | 0,10 | 0,11 | 0,09 |
| 3rd +4th generation cephalosporins | 0,08 | 0,08 | 0,08 | 0,08 | 0,09 |
| Fluoroquinolones | 0,06 | 0,07 | 0,07 | 0,08 | 0,08 |
| 1st + 2nd generation cephalosporins | 0,02 | 0,03 | 0,04 | 0,04 | 0,04 |
| Other antibiotics | 0,04 | 0,03 | 0,02 | 0,02 | 0,02 |
| Lincosamides | 0,02 | 0,02 | 0,02 | 0,02 | 0,02 |
| Polymyxins | 0,01 | 0,01 | 0,01 | 0,01 | 0,01 |
| Pleuromutiline | <0,01 | <0,01 | <0,01 | <0,01 | <0,01 |
| Total | 7,19 | 6,59 | 7,06 | 7,35 | 7,02 |
Percentage of sales volumes in relation to the total sales volume for cattle per type of utilisation for the years 2019 to 2023
| Type of utilisation | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|
| Other | 2,7 % | 3,0 % | 3,9 % | 4,4 % | 4,2 % |
| Mast | 7,1 % | 7,0 % | 7,9 % | 8,0 % | 7,4 % |
| Fattened calf | 2,0 % | 1,9 % | 3,0 % | 3,6 % | 3,1 % |
| Milk | 6,4 % | 6,6 % | 7,2 % | 9,3 % | 10,1 % |
| Suckler cow | 0,4 % | 0,4 % | 0,5 % | 0,6 % | 0,7 % |
| Breeding | 3,2 % | 0,7 % | 0,3 % | 0,7 % | 0,8 % |
| Total | 21,7 % | 19,7 % | 22,9 % | 26,5 % | 26,3 % |
Poultry delivery quantities
The following table shows the reported release quantities for poultry per active substance group in tonnes. Analogous to the previous chapters, the table below shows the release quantities in per cent by type of use for poultry.
Release quantities for the animal species poultry per active substance group in tonnes for the years 2019 to 2023
| Active substance group | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|
| Macrolides | 0,42 | 0,50 | 0,50 | 0,44 | 0,54 |
| Penicillins with extended spectrum | 0,81 | 0,80 | 0,79 | 0,57 | 0,49 |
| Polymyxine | 0,27 | 0,31 | 0,28 | 0,24 | 0,31 |
| Sulfonamides | 0,19 | 0,14 | 0,13 | 0,16 | 0,17 |
| Tetracyclines | 0,27 | 0,32 | 0,15 | 0,15 | 0,12 |
| Fluoroquinolones | 0,05 | 0,07 | 0,05 | 0,05 | 0,08 |
| Trimethoprim and derivatives | 0,04 | 0,03 | 0,03 | 0,03 | 0,03 |
| Amphenicole | <0,01 | 0,01 | 0,01 | 0,02 | 0,01 |
| Aminoglycosides | 0,02 | 0,03 | 0,02 | 0,02 | 0,01 |
| Beta-lactamase sensitive penicillins | 0,01 | 0,01 | 0,01 | 0,01 | 0,01 |
| Lincosamides | 0,01 | 0,01 | 0,03 | 0,01 | 0,01 |
| Beta-lactamase resistant penicillins | 0,00 | 0,00 | 0,00 | 0,00 | <0,01 |
| 3rd +4th generation cephalosporins | 0,00 | 0,00 | <0,01 | 0,00 | 0,00 |
| Other antibiotics | <0,01 | 0,00 | 0,00 | 0,00 | 0,00 |
| Pleuromutiline | <0,01 | <0,01 | <0,01 | <0,01 | 0,00 |
| Total | 2,10 | 2,23 | 1,99 | 1,71 | 1,80 |
Percentage of the quantities sold in relation to the total quantity sold for the animal species poultry per type of utilisation for the years 2019 to 2023
| Type of utilisation | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|
| Other | <0,1 % | <0,1 % | <0,1 % | <0,1 % | <0,1 % |
| Parent animals | 0,5 % | 0,7 % | 0,5 % | 0,4 % | 0,6 % |
| Pullets | 0,1 % | 0,1 % | 0,1 % | 0,1 % | 0,2 % |
| Laying hens | 0,8 % | 0,9 % | 0,9 % | 1,0 % | 1,6 % |
| Broiler chicken | 3,3 % | 2,9 % | 2,8 % | 2,4 % | 2,3 % |
| Fattening turkey | 1,6 % | 2,0 % | 2,0 % | 2,2 % | 2,1 % |
| Total | 6,3 % | 6,7 % | 6,5 % | 6,2 % | 6,7 % |
Application data
Since this year, veterinarians in charge of in-house pharmacies have also been able to report the veterinary medicinal products they use on animals themselves. This additional data collection contributes to a more comprehensive presentation of antibiotic use. For 2023, a quantity of 0.609 tonnes of active substances was reported via the application data. This quantity was reported almost exclusively for the animal species cattle. As the reporting obligation has not yet been defined in more detail in the Veterinary Antibiotics Volume Flow Regulation and has so far only been based on Regulation (EU) 2021/578, it can be assumed that the data is currently still incomplete. It can be assumed that the data situation will improve in the coming years.
Benchmarking reports
The term benchmarking generally refers to the comparison of a result with a reference value. As part of an international research project (Guidelines for Collection, Analysis and Reporting of Farm-level Antimicrobial Use, in the Scope of Antimicrobial Stewardship, www.aacting.org), guidelines were developed for the use of antibiotics, which cover the topics of data collection, data analysis, reporting and benchmarking. Benchmarking is seen as an effective tool for raising awareness and avoiding the use of antibiotics.
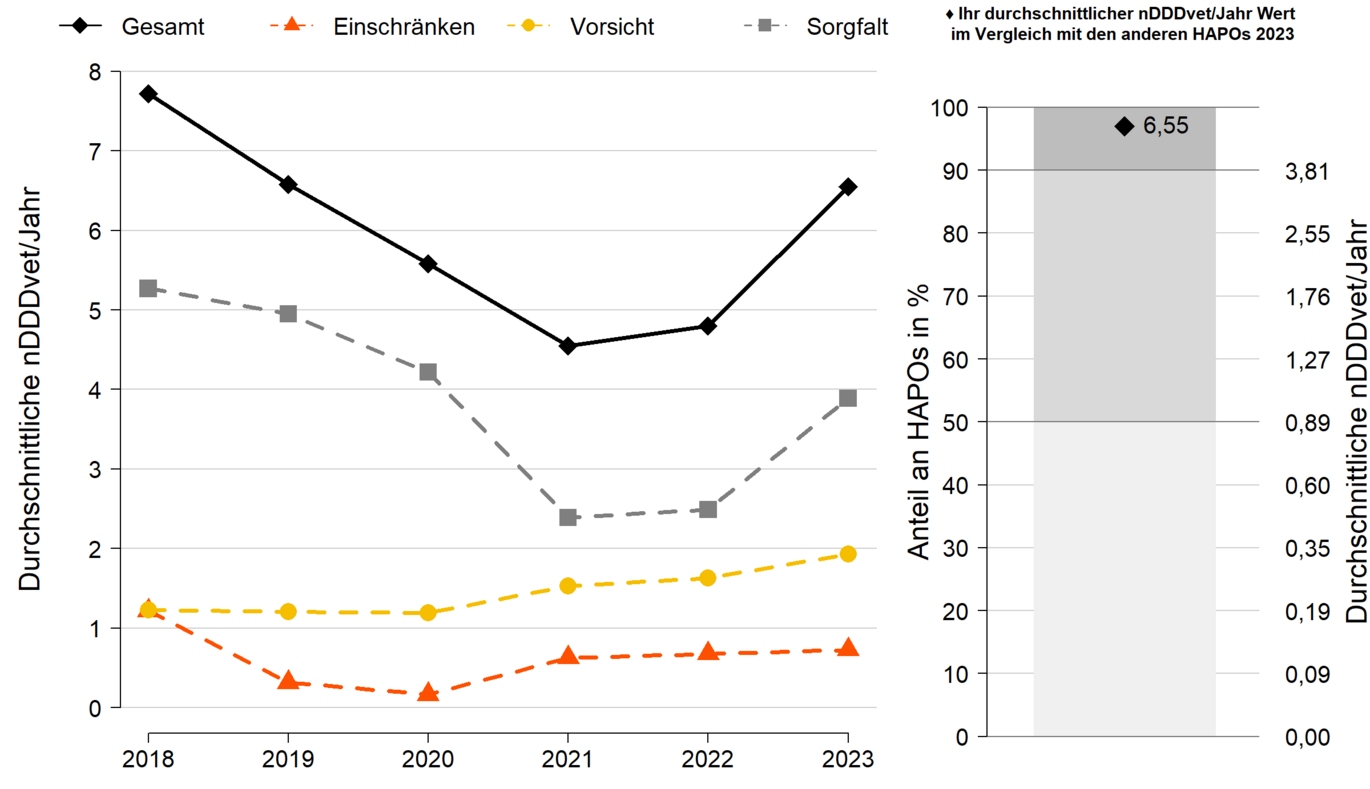
Taking this guideline into account, a benchmarking report was implemented for veterinarians managing in-house pharmacies. In addition to analysing their own antibiotic dispensing volumes over time, this also includes a comparison with other veterinarians who run in-house pharmacies (benchmarking). As an indicator for the use of antibiotics, the dispensing quantities are converted into daily doses and standardised with the respective herd size (nDDDvet/year). This results in the average number of days per year on which each animal on the farms monitored was treated.
The analyses are presented separately for the different types of animal and use. Veterinarians in charge of in-house pharmacies can download their individual report via the portal https://eservices.basg.gv.at/, where they have to report their dispensing quantities, or via the Animal Health Data Service (AHDS).
In addition, benchmarking reports on their antibiotic consumption (based on dispensing quantities) are also compiled for pig and cattle farmers, which can also be accessed via the AHDS.
- Left part of the graph
Development over time of the values of nDDDvet per year for an example HAPO, broken down into the three summarised active substance categories and overall. The categorisation of the active substances was based on the recently published EMA report (European Medicines Agency, Committee for Medicinal Products for Veterinary use (CVMP), Committee for Medicinal Products for Human Use (CHMP) 2019). - Right-hand side of the graph
Comparison of the total nDDDvet per year with the nDDDvet per year of all other veterinary in-house pharmacies for the current evaluation year. The light grey area contains the HAPOs whose average nDDDvet/year values are in the lower average of all HAPOs (lower 50 %). The dark grey area, on the other hand, contains the HAPOs whose average nDDDvet/year values are in the upper 10 %. The black square reflects the average nDDDvet/year value of the sample HAPO. If the dot is in the light grey area, this HAPO is one of the HAPOs with a low to medium average nDDDvet/year value. If it is in the dark grey area, this HAPO is one of the 10% of HAPOs with the highest nDDDvet/year values.
In the example shown, the average nDDDvet/year value is 6.55, making this HAPO one of the 10% of HAPOs with the highest nDDDvet/year values.
Animal Health Data Service (AHDS)
The new "Animal Health Data Service" (AHDS) tool from AGES has been available since September 2023 and can be accessed via the following link: https: //ahds.ages.at/. The aim of the AHDS is to link existing databases and provide targeted analyses to the various user groups. This should enable central challenges - such as reducing the use of antibiotics and improving animal health - to be monitored and evaluated in a simple and accessible way based on data. Animal owners, veterinarians in charge of in-house pharmacies and authorities can already view individualised antibiotic evaluations via the AHDS.

Discussion
The sharp decline in distribution volumes from the previous year continued this year and, at 32.54 tonnes, is the lowest figure since data collection began (for the first time for 2010). The last few years were also characterised by stronger fluctuations in sales volumes, which were partly due to stockpiling and destocking (due to uncertainty about the market situation and the (non-)availability of medicinal products).
The sales volumes of active pharmaceutical ingredients classified as "Highest Priority Critically Important Antimicrobials (HPCIA)" (WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance and World Health Organization 2017) have fluctuated between 4 and 5.78 tonnes over the last five years and stood at 4 tonnes in 2023. Over the years, HPCIAs have accounted for a relatively constant 12-13% of the total quantity.
The mg/PCU indicator, which represents a rough estimate of the quantity of antibiotics sold per kilogramme of food mass produced, fell to 34.4 mg/PCU in 2023 and was therefore 5% lower than in 2022. In absolute terms, this means a decrease of 1.8 mg/PCU. The ratio of total antibiotics purchased by the HAPO to total antibiotics dispensed was 80.9% in 2022 and 81.9% in 2023.
Based on the Austrian recording system, in which each HAPO must report the quantities dispensed per farm, animal species and type of use, it is possible to create species-specific analyses. Compared to the previous year, the release quantities for 2023 show a decrease of -0.6 mg/PCU for cattle and increases of 0.5 mg/PCU for pigs and 0.9 mg/PCU for poultry. These values only reflect a general trend and are subject to certain uncertainties. These are to be reduced in future by the additional recording of application data and other animal species in order to obtain a more accurate picture of antibiotic use in animals.
With the Animal Health Data Service (AHDS), a new evaluation platform was created in 2023 that links different databases and provides targeted analyses for the respective user groups. Cattle and pig farmers and veterinarians who manage in-house pharmacies can view their antibiotic analyses via the AHDS. Easy access to these analyses is intended to make a further contribution to raising awareness regarding the use of antibiotics.
Authors
Reinhard Fuchs
Elisabeth Reitbauer
Dr Gernot Resch
Univ.-Doz. DI Dr Klemens Fuchs
Austrian Agency for Health and Food Safety GmbH
Department of Integrative Risk Assessment, Data and Statistics
Zinzendorfgasse 27, 8010 Graz
With the kind support of the AGES Medical Market Surveillance Division
- European Medicines Agency. 2011. "Trends in the Sales of Veterinary Antimicrobial Agents in Nine European Countries (2005-2009)." EMA/238630/2011.
- European Medicines Agency, Committee for Medicinal Products for Veterinary use (CVMP), Committee for Medicinal Products for Human Use (CHMP). 2019. "Categorisation of Antibiotics in the European Union." EMA/CVMP/CHMP/682198/2017.
- European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption, 2022. "Sales of veterinary antimicrobial agents in 31 European countries in 2022." EMA/299538/2023.
- European Union. 2020. "Farm to Fork Strategy - for a Fair, Healthy and Environmentally-Friendly Food System. " https://food.ec.europa.eu/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf
- R Core Team. 2024. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.
- WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance, and World Health Organisation. 2017. Critically Important Antimicrobials for Human Medicine: Ranking of Antimicrobial Agents for Risk Management of Antimicrobial Resistance Due to Non-Human Use.
- World Health Organization Collaborating Centre for Drug Statistics Methodology. n.d. "WHOCC - ATCvet." ATCvet System for Classification of Veterinary Medicines. https://www.whocc.no/atcvet/
AB Antibiotics
AHDS Animal Health Data Service
AMEG Antimicrobial Advice Ad Hoc Expert Group
EMA European Medicines Agency
ESVAC European Surveillance of Veterinary Antimicrobial Consumption
HAPO Veterinary in-house pharmacy
WHO World Health Organisation
HPCIA Highest Priority Critically Important Antimicrobials
PCU Population Correction Unit
In Österreich wurden im Jahr 2020 insgesamt 43,65 Tonnen Antibiotika für den Einsatz in der Veterinärmedizin verkauft. Im Vergleich zum Jahr 2019 kam es zu einer Zunahme der verkauften Gesamtmenge um 3,15 Tonnen. Das entspricht einer relativen Zunahme von 7,8 %.
Mengenbericht 2020
In Österreich wurden im Jahr 2019 insgesamt 40,69 Tonnen Antibiotika für den Einsatz in der Veterinärmedizin verkauft. Im Vergleich zum Jahr 2018 kam es zu einer Abnahme der verkauften Gesamtmenge um 7,32 Tonnen. Das entspricht einer relativen Abnahme von 15,2 %. Die Gesamtvertriebsmenge an antimikrobiell wirksamen Substanzen für Nutztiere ist seit 2015 um 13 % zurückgegangen.
In Österreich wurden im Jahr 2018 insgesamt 49,85 Tonnen Antibiotika für den Einsatz in der Veterinärmedizin verkauft. Im Vergleich zum Jahr 2017 kam es zu einer Zunahme der verkauften Gesamtmenge um 5,23 Tonnen. Das entspricht einer relativen Zunahme von 11,72 %. Die Gesamtvertriebsmenge an antimikrobiell wirksamen Substanzen für Nutztiere ist seit 2014 um 7 % zurückgegangen.
In Österreich wurden im Jahr 2017 insgesamt 44,61 Tonnen Antibiotika für den Einsatz in der Veterinärmedizin verkauft. Im Vergleich zum Jahr 2016 kam es zu einer Zunahme der verkauften Gesamtmenge um 0,2 Tonnen. Das entspricht einer relativen Zunahme um 0,5%. Die Gesamtvertriebsmenge an antimikrobiell wirksamen Substanzen für Nutztiere ist seit 2013 jedoch um 19 % zurückgegangen.
In Österreich wurden im Jahr 2016 insgesamt 44,41 Tonnen Antibiotika für den Einsatz in der Veterinärmedizin verkauft. Im Vergleich zum Jahr 2015 kam es zu einer Abnahme der verkauften Gesamtmenge um 4,37 Tonnen. Das entspricht einer relativen Abnahme um 8,96 %. Nach wie vor werden mit 94 % mengenmäßig am meisten Antibiotika für die systemische Anwendung (QJ01) verkauft.
In Österreich wurden im Jahr 2015 insgesamt 48,78 Tonnen Antibiotika für den Einsatz in der Veterinärmedizin verkauft. Im Vergleich zum Jahr 2014 kam es zu einer signifikanten Abnahme der verkauften Gesamtmenge um rund 4,89 Tonnen. Das entspricht einer relativen Abnahme um rund 9,1 %.
Für die Erfassung der Daten des Jahres 2015 müssen neben den pharmazeutischen Firmen und Großhändlern auch die hausapothekenführenden Tierärztinnen und Tierärzte ihre Daten direkt über die Homepage der Medizinmarktaufsicht der AGES in die Datenbank hochladen. Somit ist es nun erstmals möglich eine Zuordnung der Menge der antimikrobiell wirksamen Substanzen zu einzelnen Tierarten vorzunehmen. Dementsprechend entfallen rund 75,8 % der abgegebenen Antibiotika auf die Nutztierspezies Schwein, rund 17 % auf Rinder und 6,8 % auf Geflügel.
In Österreich wurden im Jahr 2014 insgesamt 53,67 Tonnen Antibiotika für den Einsatz in der Veterinärmedizin verkauft. Im Vergleich zum Jahr 2013 kam es zu einer Abnahme der verkauften Gesamtmenge um rund 1,31 Tonnen. Das entspricht einer relativen Abnahme um rund 2,4 %. Dies ist zu einem geringen Teil sicher auch auf die Umstellung auf ein neues Erfassungssystem zurückzuführen.
Für die Erfassung der Daten des Jahres 2014 wurde erstmals auf ein neues System umgestellt: Die pharmazeutischen Firmen und Großhändler laden ihre Daten nun direkt über die Homepage der Medizinmarktaufsicht der AGES in die Datenbank. Aus diesen Daten wird die insgesamt vertriebene Menge an Wirksubstanz in Tonnen berechnet. Eine Zuordnung der Menge der antimikrobiell wirksamen Substanzen zu einzelnen Tierarten findet nicht statt.
Seit 2010 wird mittels eines von der EMA (European Medical Agency) entwickelten Verfahrens von der AGES/DSR im Auftrag des BMG eine lückenlose Erfassung der verkauften Mengen von Tierarzneimitteln für die Nutztiermedizin, die Antibiotika enthalten, bei allen in Österreich tätigen Arzneimittelfirmen durchgeführt. Die Erhebung ergab, dass in Österreich 2013 insgesamt 54,98 Tonnen Antibiotika an TierärztInnen für den Einsatz in der Nutztiermedizin verkauft wurden. Verglichen mit 2012 war das eine Steigerung um 3,3 Prozent.
Wie schon in den Jahren 2010 und 2011 wurden auch für das Jahr 2012 mittels eines von der EMA (European Medical Agency) entwickelten Verfahrens von der AGES/DSR im Auftrag des BMG eine lückenlose Erfassung der verkauften Mengen von Tierarzneimitteln für die Nutztiermedizin, die Antibiotika enthalten, bei allen in Österreich tätigen Arzneimittelfirmen durchgeführt. Die Studie ergab, dass in Österreich 2012 insgesamt 53,22 Tonnen Antibiotika an TierärztInnen für den Einsatz in der Veterinärmedizin verkauft wurden. Verglichen mit 2011 war das eine Reduktion um 0,22 Tonnen oder -0,41 %.
Wie schon für das Jahr 2010 wurden auch für das Jahr 2011 mittels eines von der EMA (European Medical Agency) entwickelten Verfahrens von der AGES/DSR im Auftrag des BMG eine lückenlose Erfassung der verkauften Mengen von Tierarzneimitteln für die Nutztiermedizin, die Antibiotika enthalten, bei allen in Österreich tätigen Arzneimittelfirmen durchgeführt. Die Studie ergab, dass in Österreich 2011 insgesamt 53,44 Tonnen Antibiotika an TierärztInnen für den Einsatz in der Veterinärmedizin verkauft wurden. Verglichen mit 2010 war das eine Reduktion um 9,39 Tonnen oder -15 %.
In Österreich wurden 2010 insgesamt 62,83 Tonnen Antibiotika zur Anwendung bei den Nutztierarten Rind, Schwein, Geflügel, Schaf und Ziege verkauft. Die überwiegende Menge der Antibiotika in der Veterinärmedizin entfiel auf oral oder als Injektion zu verabreichende, systemisch wirksame Substanzen.
Last updated: 08.10.2025
automatically translated