Good data quality is essential for a better understanding of the relationship between antimicrobial resistance and antimicrobial use. Pharmaceutical companies and wholesalers are required to report their sales of veterinary antimicrobial products. Further, veterinary pharmacies must report their dispense data. The collection of sales data by pharmaceutical companies, wholesalers and veterinarians is regulated by the national law on animal drug control (Veterinär-Antibiotika-Mengenströme-Verordnung). In this report, sales and dispense data of antimicrobial agents, which are licensed for food-producing animals, are presented for the year 2021 and compared with those of previous years. The total sales data of antimicrobial agents for food-producing animals has decreased by 10,5% compared to 2020 and lies at 39,07 tons in 2021. The sales data of “HPCIA” (according to the World Health Organization) has decreased by 19% from 5,72 t in 2020 to 4,64 t in 2021. Over the years, HPCIA have a relatively constant share of around 12% of the total sales. In general, the sales volumes of the last few years show fluctuations, which are partly attributable to higher stock levels. The underlying animal population shows only minor fluctuations over the last few years, therefore changes in the sales volume are not likely attributable to a change in the number of animals. Veterinary pharmacies are obliged to report the animal category for which the antimicrobials are dispensed. This allows the assignment of the dispense data of antimicrobial agents to animal species. For a better comparison of the amounts of veterinary antimicrobial agents, the European Medicines Agency (EMA) proposed to link these data to the animal demographics in each country. They established the PCU as a normalization factor for the data. The PCU is a technical unit and 1 PCU equals 1 kg body mass. This results in standardized values for pigs of 61 mg/PCU (-7,8 mg/PCU compared to 2020), for cattle of 16,7 mg/PCU (+1,1 mg/PCU) and for poultry of 22 mg/PCU (-3,1 mg/PCU). These figures are, however, derived from the dispense data as the exact amounts used at the farms (use data) are not reported. Therefore, they are subject to a greater degree of uncertainty.
Introduction
The Veterinary Antibiotics Flow Regulation (BGBl. II No. 83/2014, last amended by BGBl. II No. 127/2022) provides the legal basis for a system to record the distribution and consumption of antibiotics in the veterinary sector in Austria. According to §4(2), AGES has the task to prepare a report on the evaluation of the data and to publish it via the Federal Ministry of Social Affairs, Health, Care and Consumer Protection.
This report presents the distribution volumes of antibiotics approved for use in veterinary medicine for livestock from 2017 to 2021 and the dispensing volumes from 2016 to 2021.
Sales volumes
For the years 2010-2013, the sales volumes of veterinary medicinal products containing antibiotics were reported electronically to AGES by the manufacturers, marketing authorization holders (depositors) and pharmaceutical wholesalers via a document specified by the EMA (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption 2021) and prepared by AGES. With the entry into force of the Veterinary Antibiotics Flow Regulation, a new system was introduced for the first time for the collection of data for 2014; since then, manufacturers, marketing authorization holders (distributors) and pharmaceutical wholesalers have been uploading their data directly into the database via the homepage of the AGES Medical Market Authority. From this data, the total quantity of active ingredient sold is calculated in tons.
Delivery quantities
In addition, since 2016, veterinarians managing in-house pharmacies have had to upload their data on the dispensing of antibiotics for use on livestock (except horses, see Veterinary Antibiotics Flow Regulation §7(2)) for the respective previous year either independently or electronically via recognized reporting points in the new system in accordance with the Veterinary Antibiotics Flow Regulation. These data include, among other things, information on the type of animal and type of use. This makes it possible to allocate the quantity of antimicrobially active substances to individual animal species as of the data for 2015.
ATCvet categories
For the classification of active substances, the ATCvet system (World Health Organization Collaborating Centre for Drug Statistics Methodology, n.d.) is used in analogy to the ATC system used in human medicine (see table). For antibiotics expressed in international units, conversion factors have been provided by the EMA (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption 2021).
| Category | ATCvet Code |
|---|---|
| AB for intestinal use | QA07AA; QA07AB |
| AB for intrauterine use | QG01AA; QG01AE; QG01BA; QG01BE; GQ51AA; QG51AG |
| AB for systemic use | QJ01 |
| AB for intramammary use | QJ51 |
| Antiparasitics | QP51AG |
Categories considered or associated ATCvet codes (World Health Organization Collaborating Centre for Drug Statistics Methodology, n.d.; European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption 2021).
Quantities in this report differ from published reports in previous years due to the adoption of an EMA proposal for further harmonization (adjustment of conversion factors to determine pure substance quantity) of veterinary antibiotic data. Therefore, corrections have been made to the data for all years.
Results of the sales volume survey
Compared to 2020, there was a decrease of 4.58 tons in the total amount sold in 2021. This corresponds to a relative decrease of 10.5%.
| Year | Sales volume | Difference (absolute) | Difference (relative) |
|---|---|---|---|
| 2017 | 42,74 | - | - |
| 2018 | 47,83 | 5,09 | 11,91 |
| 2019 | 40,51 | -7,32 | -15,31 |
| 2020 | 43,65 | 3,14 | 7,74 |
| 2021 | 39,07 | -4,58 | -10,48 |
Looking at the type of application, oral preparations for the treatment of individual animals or groups of animals - these include powders, tablets and pastes - are far ahead of the other forms of application in 2021 as well, with 31.7 tons (81.1%). Parenterally applied preparations are in second place with 5.4 tons (13.8%), followed by the group of intramammary applications, to which dry preparations were also assigned, with 1.02 tons (2.6%). Feeding drug premixes (premix) used orally for herd treatment account for 0.87 tons (2.2%) in terms of volume.
In terms of sales volumes broken down by groups of active ingredients, tetracycline remains in first place with 19.3 metric tons (49.4%), followed by extended-spectrum penicillins with 6.3 metric tons (16.1%), sulfonamides with 3.7 metric tons (9.5%) and macrolides with 2.5 metric tons (6.4%). The classification of the active ingredients into active ingredient groups was carried out analogously to the specifications of the EMA (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption 2021). The group "Other antibiotics" includes, among others, "Rifaximin" and "Spectinomycin".
Evaluations of the active ingredient groups of macrolides, fluoroquinolones, "3rd and 4th generation cephalosporins" and polymyxins, which are classified by the WHO as antibiotics of the highest importance for human medicine, are also presented separately in section 3.3.
Volumes sold by active ingredient group for the years 2017 to 2021 and the difference between the years 2021 and 2020 in tons.
| Active ingredient group | 2017 | 2018 | 2019 | 2020 | 2021 | Diff. |
|---|---|---|---|---|---|---|
| Tetracyclines | 23,72 | 25,75 | 19,72 | 22,11 | 19,30 | -2,81 |
| Penicillins with extended spectrum | 5,70 | 6,94 | 6,60 | 7,35 | 6,26 | -1,09 |
| Sulfonamides | 3,37 | 4,30 | 3,92 | 3,46 | 3,66 | 0,20 |
| Macrolides | 2,96 | 3,28 | 2,97 | 3,47 | 2,48 | -0,99 |
| Beta-lactamase sensitive penicillins | 1,57 | 1,69 | 1,56 | 1,58 | 1,64 | 0,06 |
| Polymyxins | 1,67 | 1,79 | 1,53 | 1,54 | 1,47 | -0,07 |
| Aminoglycosides | 1,12 | 1,18 | 1,28 | 1,29 | 1,32 | 0,03 |
| Trimethoprim and derivatives | 0,67 | 0,86 | 0,78 | 0,69 | 0,73 | 0,04 |
| Fluoroquinolones | 0,47 | 0,49 | 0,46 | 0,48 | 0,46 | -0,02 |
| Beta-lactamase resistant penicillins | 0,43 | 0,42 | 0,41 | 0,38 | 0,41 | 0,03 |
| Amphenicols | 0,41 | 0,42 | 0,47 | 0,42 | 0,39 | -0,03 |
| Pleuromutiline | 0,23 | 0,30 | 0,39 | 0,41 | 0,33 | -0,08 |
| 3rd +4th generation cephalosporins | 0,22 | 0,22 | 0,22 | 0,23 | 0,23 | 0,00 |
| Lincosamide | 0,06 | 0,06 | 0,10 | 0,09 | 0,20 | 0,11 |
| other antibiotics | 0,10 | 0,10 | 0,07 | 0,09 | 0,16 | 0,07 |
| 1st + 2nd generation cephalosporins | 0,04 | 0,04 | 0,03 | 0,04 | 0,05 | 0,01 |
| Total | 42,74 | 47,83 | 40,51 | 43,65 | 39,07 | -4,58 |
Antibiotics for intestinal use
For antibiotics for intestinal use (ATCvet QA07), there has been a decrease in the quantity sold in 2021 compared to 2020.
Volumes sold by active ingredient group (for intestinal use) for 2017 to 2021 and the difference between 2021 and 2020 in tons.
| Active ingredient group | 2017 | 2018 | 2019 | 2020 | 2021 | Diff. |
|---|---|---|---|---|---|---|
| Polymyxins | 1,67 | 1,79 | 1,53 | 1,54 | 1,47 | -0,07 |
| Aminoglycosides | 0,11 | 0,17 | 0,23 | 0,27 | 0,32 | 0,05 |
| Total | 1,78 | 1,96 | 1,76 | 1,81 | 1,79 | -0,02 |
Antibiotics for intramammary use
The following table shows the quantities of intramammary antibiotics (ATCvet QJ51) applied, broken down according to dryer and other preparations used during lactation. A slight increase compared to the previous year can be observed here.
Sold quantities by active ingredient group (for intramammary use) for the years 2017 to 2021 and the difference between the years 2021 and 2020 in tons.
| Active ingredient group | 2017 | 2018 | 2019 | 2020 | 2021 | Diff. |
|---|---|---|---|---|---|---|
| Beta-lactamase sensitive penicillins | 0,31 | 0,33 | 0,32 | 0,35 | 0,36 | 0,01 |
| 1st + 2nd generation cephalosporins | 0,03 | 0,03 | 0,02 | 0,03 | 0,04 | 0,01 |
| Aminoglycosides | 0,01 | 0,02 | 0,01 | 0,02 | 0,02 | 0,00 |
| Lincosamide | 0,01 | 0,01 | 0,02 | 0,01 | 0,02 | 0,01 |
| 3rd +4th generation cephalosporins | 0,03 | 0,03 | 0,03 | 0,03 | 0,02 | -0,01 |
| Penicillins with extended spectrum | 0,03 | 0,01 | 0,01 | 0,01 | 0,01 | 0,00 |
| Trimethoprim and derivatives | <0,01 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 |
| Sulfonamides | <0,01 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 |
| Beta-lactamase resistant penicillins | 0,02 | 0,00 | 0,00 | 0,00 | 0,00 | 0,00 |
| Subtotal 'During lactation | 0,44 | 0,43 | 0,42 | 0,45 | 0,48 | 0,03 |
| Beta-lactamase resistant penicillins | 0,37 | 0,38 | 0,37 | 0,37 | 0,38 | 0,01 |
| Beta-lactamase sensitive penicillins | 0,08 | 0,08 | 0,08 | 0,08 | 0,07 | -0,01 |
| Aminoglycosides | 0,04 | 0,04 | 0,04 | 0,04 | 0,03 | -0,01 |
| Penicillins with extended spectrum | 0,04 | 0,03 | 0,02 | 0,00 | 0,02 | 0,02 |
| 1st + 2nd generation cephalosporins | 0,01 | 0,01 | 0,01 | 0,01 | 0,01 | 0,00 |
| other antibiotics | 0,01 | <0,01 | <0,01 | 0,01 | 0,01 | 0,00 |
| 3rd +4th generation cephalosporins | 0,01 | 0,01 | 0,01 | 0,01 | 0,01 | 0,00 |
| Subtotal 'dryer' | 0,55 | 0,54 | 0,53 | 0,51 | 0,54 | 0,03 |
| Total | 0,99 | 0,98 | 0,94 | 0,96 | 1,02 | 0,03 |
Antibiotics for systemic use
The following two tables show the sales volumes of antibiotics for systemic use (ATCvet QJ01) by active ingredient group and application form. The greatest decrease can be seen in the tetracyclines. With regard to the form of application, a larger decrease is recorded for the orally administered antibiotics.
Volumes sold by active ingredient group (for systemic use) for the years 2017 to 2021 and the difference between the years 2021 and 2020 in tons.
| Active ingredient group | 2017 | 2018 | 2019 | 2020 | 2021 | Diff. |
|---|---|---|---|---|---|---|
| Tetracyclines | 23,67 | 25,69 | 19,67 | 22,05 | 19,21 | -2,84 |
| Penicillins with extended spectrum | 5,59 | 6,87 | 6,53 | 7,32 | 6,20 | -1,12 |
| Sulfonamides | 3,37 | 4,30 | 3,92 | 3,46 | 3,66 | 0,20 |
| Macrolides | 2,96 | 3,28 | 2,97 | 3,47 | 2,48 | -0,99 |
| Beta-lactamase sensitive penicillins | 1,18 | 1,28 | 1,16 | 1,15 | 1,21 | 0,06 |
| Aminoglycosides | 0,96 | 0,96 | 1,00 | 0,96 | 0,94 | -0,02 |
| Trimethoprim and derivatives | 0,67 | 0,86 | 0,78 | 0,69 | 0,73 | 0,04 |
| Fluoroquinolones | 0,47 | 0,49 | 0,46 | 0,48 | 0,46 | -0,02 |
| Amphenicols | 0,41 | 0,42 | 0,47 | 0,42 | 0,39 | -0,03 |
| Pleuromutiline | 0,23 | 0,30 | 0,39 | 0,41 | 0,33 | -0,08 |
| 3rd +4th generation cephalosporins | 0,18 | 0,18 | 0,18 | 0,19 | 0,19 | 0,00 |
| Lincosamide | 0,05 | 0,05 | 0,08 | 0,08 | 0,18 | 0,10 |
| Other antibiotics | 0,10 | 0,10 | 0,07 | 0,08 | 0,15 | 0,07 |
| Total | 39,83 | 44,77 | 37,67 | 40,77 | 36,13 | -4,64 |
Volumes sold by application form (for systemic use) for the years 2017 to 2021 and the difference of the years 2021 and 2020 in tons.
| Application form | 2017 | 2018 | 2019 | 2020 | 2021 | Diff. |
|---|---|---|---|---|---|---|
| Oral | 33,00 | 37,38 | 30,95 | 34,13 | 29,89 | -4,24 |
| Parenteral | 5,53 | 5,90 | 5,49 | 5,50 | 5,38 | -0,12 |
| Premix | 1,30 | 1,49 | 1,23 | 1,14 | 0,87 | -0,27 |
| Total | 39,83 | 44,77 | 37,67 | 40,77 | 36,13 | -4,64 |
Antibiotics for intrauterine use
The sales volumes of antibiotics for intrauterine use (ATCvet QG01, QG51) are shown per active ingredient group in the following table. These show a slight increase compared to the previous year only for tetracyclines.
Volumes sold by active ingredient group (for intrauterine use) for the years 2017 to 2021 and the difference between the years 2021 and 2020 in tons.
| Active ingredient group | 2017 | 2018 | 2019 | 2020 | 2021 | Diff. |
|---|---|---|---|---|---|---|
| Tetracyclines | 0,06 | 0,05 | 0,06 | 0,06 | 0,08 | 0,02 |
| Beta-lactamase resistant penicillins | 0,04 | 0,04 | 0,04 | 0,02 | 0,02 | 0,00 |
| Penicillins with extended spectrum | 0,04 | 0,04 | 0,04 | 0,02 | 0,02 | 0,00 |
| Total | 0,14 | 0,13 | 0,14 | 0,10 | 0,13 | 0,03 |
Antibiotics of utmost importance for human medicine
The active ingredient groups macrolides, fluoroquinolones, 3rd and 4th generation cephalosporins and also the group of polymyxins (including colistin) are classified by the WHO as so-called Highest Priority Critically Important Antimicrobials (HPCIA) due to their status (WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance and World Health Organization 2017). WHO also includes 5th generation cephalosporins, ketolides, and glycopeptides among HPCIA; however, these do not have sales volumes. The volumes of these drug groups sold between 2017 and 2021 are shown in the following tables. There has been a decrease here, particularly for macrolides.
Sold quantities of the antibiotics of very high importance for human medicine (HPCIA) for the years 2017 to 2021 and the difference between the years 2021 and 2020 in tons.
| Active ingredient group | 2017 | 2018 | 2019 | 2020 | 2021 | Diff. |
|---|---|---|---|---|---|---|
| Macrolides | 2,96 | 3,28 | 2,97 | 3,47 | 2,48 | -0,99 |
| Polymyxins | 1,67 | 1,79 | 1,53 | 1,54 | 1,47 | -0,07 |
| Fluoroquinolones | 0,47 | 0,49 | 0,46 | 0,48 | 0,46 | -0,02 |
| 3rd +4th generation cephalosporins | 0,22 | 0,22 | 0,22 | 0,23 | 0,23 | 0,00 |
| Total | 5,32 | 5,78 | 5,17 | 5,72 | 4,64 | -1,08 |
Standardized sales quantities
In the previous chapters, the purely surveyed sales volumes were compared with each other over the last few years. No normalization was performed on the basis of the respective animals kept (animal populations per year). In the ESVAC reports, in order to take into account the different animal demographics of the countries, the Population Correction Unit (PCU) was defined, which is calculated from herd and slaughter data, as well as imports and exports. For more information on the calculation of the PCU, see Annex 3 of the report "Trends in the sales of veterinary antimicrobial agents in nine European countries: 2005-2009" (European Medicines Agency 2011).
The unit mg/PCU is a technical quantity used to compare quantities of different animal species, countries or years; 1 PCU = 1 kg. This normalization factor shows only minor fluctuations for Austria over the last few years. This means that the changes in quantities cannot generally be explained by higher or lower animal numbers in the respective years. In the following figure, the sales volumes are shown normalized on the basis of the PCU. Here, a reduction of 11% can be seen in 2021 compared to the previous year.
Sales volumes in the pet sector
The distribution quantities presented so far exclusively include veterinary medicinal products that are approved for at least one farm animal species (or horses). Recently, all veterinary medicinal products with antimicrobial active ingredients have been reported in the distribution quantity report, including those that are only approved for pet animals (dogs, cats, etc.). The quantities in recent years are listed in the following table and range from 500 to 610 kilograms.
| Year | Quantity distributed | Difference (absolute) | Difference (relative) |
|---|---|---|---|
| 2017 | 0,59 | - | - |
| 2018 | 0,59 | <0,01 | -0,73 |
| 2019 | 0,59 | <0,01 | 0,07 |
| 2020 | 0,51 | -0,08 | -13,39 |
| 2021 | 0,61 | 0,10 | 18,78 |
Veterinary home pharmacies
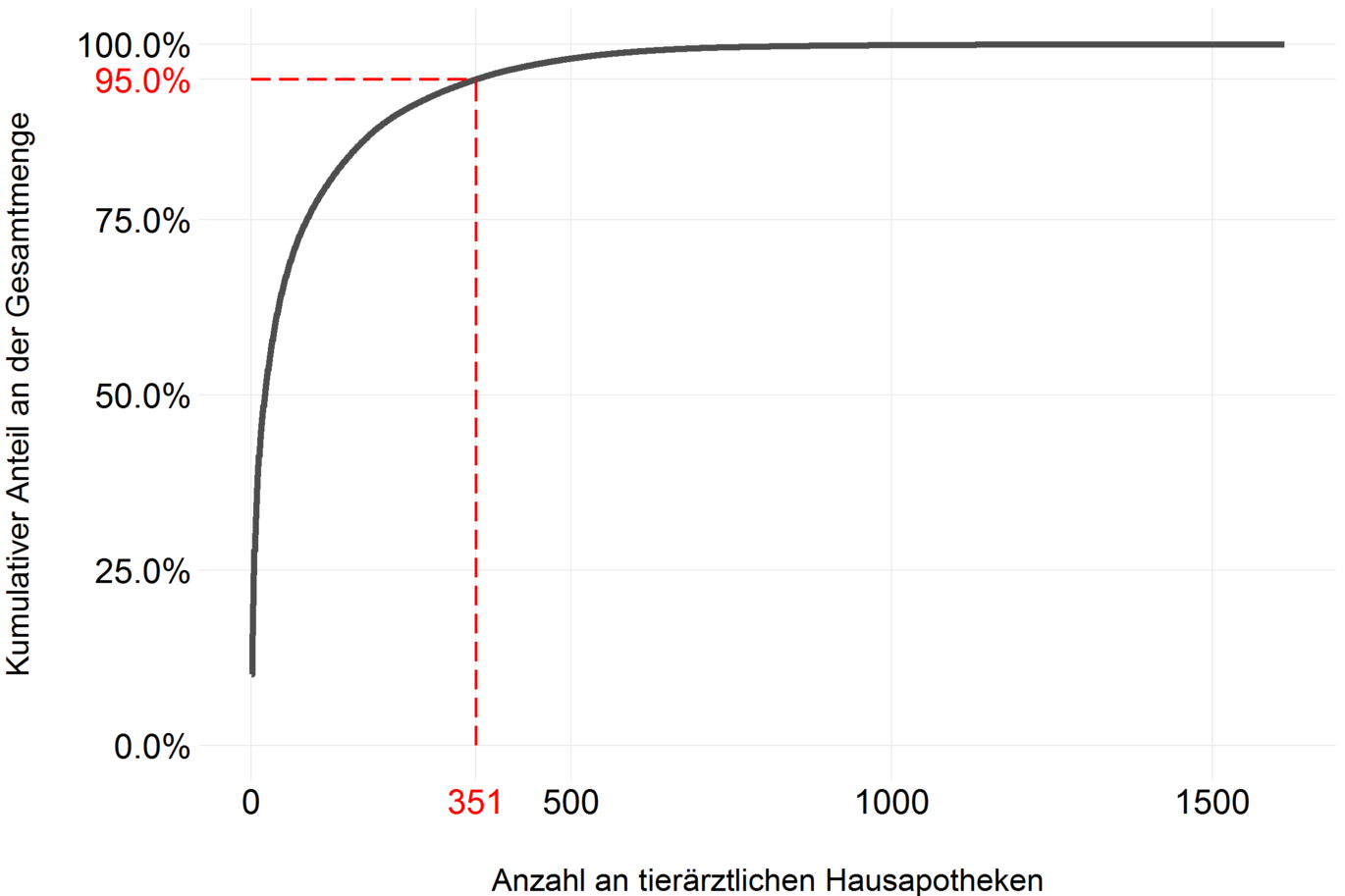
As mentioned in the Data and Method chapter, a new electronic data collection system has been developed for recording sales volumes as of 2014. Among other things, pharmaceutical companies and wholesalers must report to which home apothecary (HAPO) how many packages of which product were sold. In Austria, 1752 HAPOs were reported in 2021, of which 1613 also purchased antibiotics in 2021.
In order to examine how the quantities of antibiotics sold are distributed among the individual HAPOs, the cumulative distribution of the quantity shares per HAPO of the total quantity is shown in the following figure. The very steep slope of the curve suggests that very few HAPOs purchase very large quantities of antibiotics. The red dashed line indicates that 95% of the antibiotics were sold to 351 HAPOs (about 20%). Conversely, this means that 80% of HAPOs purchase only 5% of the total.
Results of the delivery quantity survey
In the dispensing report, veterinarians managing in-house pharmacies must indicate which antibiotics were dispensed to which farms, and in what quantities. A total of 571 of 1752 HAPOs complied with this reporting obligation for the reporting year 2021.
In order to be able to check the completeness of the dispensing data, starting this year, veterinarians in charge of in-house pharmacies who are not obliged to submit a dispensing report according to the Veterinary Antibiotics Flow Regulation §7(2) must submit an empty dispensing report (see Veterinary Antibiotics Flow Regulation §7(3)). This year, 898 HAPOs have carried out such a declaration.
Of the above-mentioned 351 HAPOs (top 95%), 315 have made a delivery notification or empty delivery notification. In total, about 31 tons of antibiotics dispensed to farms were reported. The difference of about 8.1 tons (21%) to the reporting of manufacturers, depositors and drug wholesalers may have different reasons (e.g..: Use by veterinarian, dispensing to non-reportable species, stockpiling, non-reporting). The following table shows the dispensing and distribution quantities, as well as the respective differences and proportions.
Dispensing quantity, distribution quantity and the share of dispensing quantities in the distribution quantity in absolute and relative terms per year.
| Reporting year | Quantity sold | Sales quantity | Difference: absolute | relative |
|---|---|---|---|---|
| 2017 | 34,42 | 42,74 | 8,32 | 19,48 |
| 2018 | 37,40 | 47,83 | 10,44 | 21,82 |
| 2019 | 33,17 | 40,51 | 7,34 | 18,11 |
| 2020 | 33,51 | 43,65 | 10,14 | 23,23 |
| 2021 | 30,96 | 39,07 | 8,11 | 20,75 |
Species-specific evaluations
In addition to indicating to which farms antibiotics were dispensed, the veterinarians in charge of in-house pharmacies must also report for which animal species and type of use the antibiotics were dispensed. In the following figure, it can be seen that in 2021, 71% of the quantity was dispensed for the animal species pig, followed by cattle with 23% and poultry with 6%.
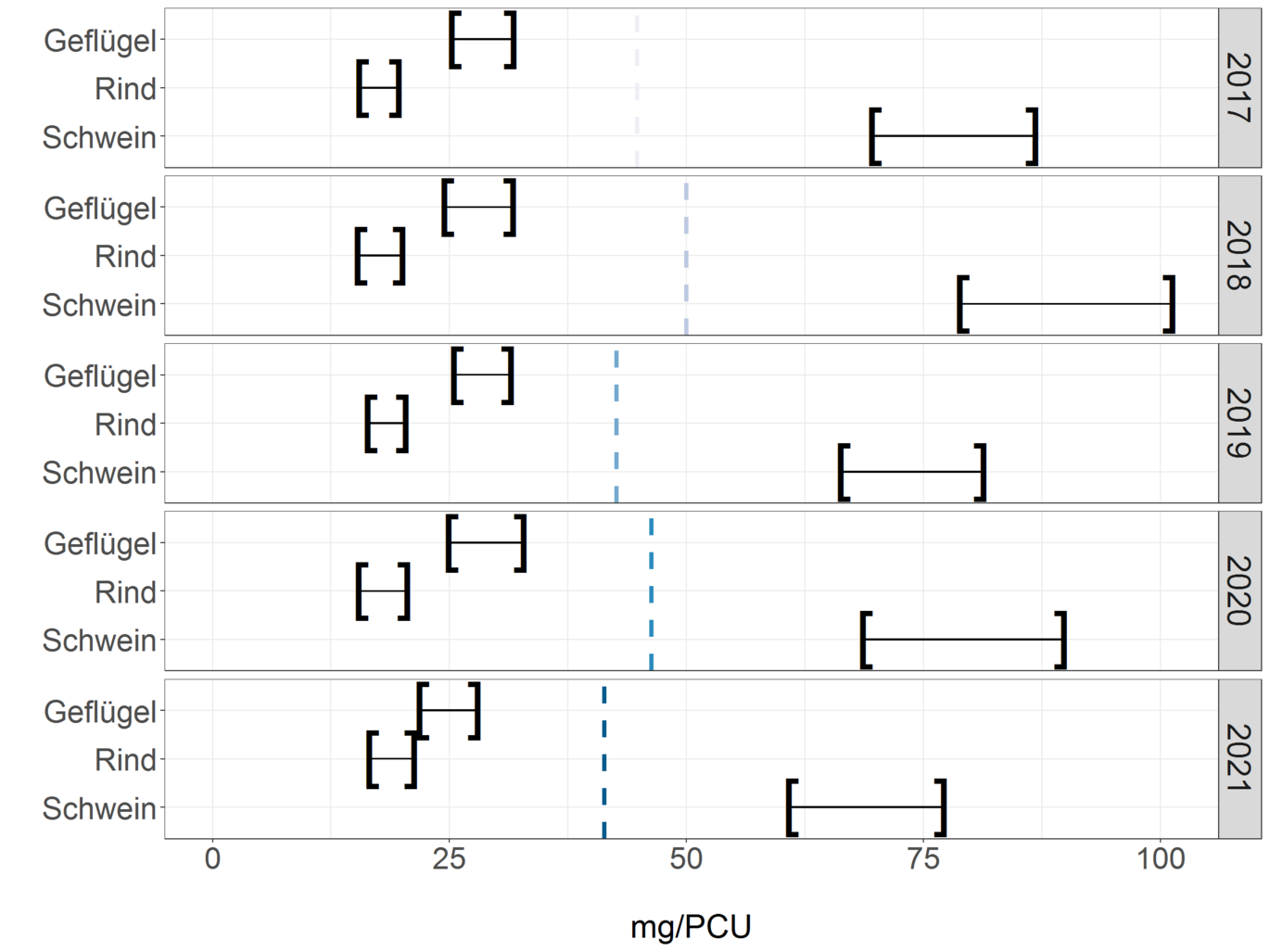
Since the animal populations and slaughter numbers of different animal species differ from one another, the release quantities are presented in normalized form, as in the ESVAC report (European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption 2021).
In the following figure, the dispensing quantities for pigs, cattle and poultry are shown in mg/PCU. The left parenthesis in each case indicates the normalized reported release quantity. As can be seen in the table, the sum of the reported release quantities is 21% lower than the total distribution quantity. This difference was taken into account for the respective animal species and extrapolated in the right brackets in the figure. The values shown in the graph can be seen in the table. These figures are currently subject to greater uncertainty, as AB use in horses and pets is not taken into account here, and the proportion of sales for use in cattle, pigs and poultry is not identical.
Normalized dispensing volumes based on PCU per animal species of pigs, cattle, and poultry for 2017 to 2021. Column mg/PCU corresponds to the normalized reported dispensing volumes; column mg/PCU (extrapolated) reflects the values extrapolated taking into account the reporting difference to the distribution volume.
| Year | Animal species | mg/PCU | mg/PCU (extrapolated) |
|---|---|---|---|
| 2017 | Poultry | 25,4 | 31,6 |
| 2017 | Beef | 15,7 | 19,5 |
| 2017 | Pork | 69,7 | 86,6 |
| 2018 | Poultry | 24,7 | 31,5 |
| 2018 | Beef | 15,5 | 19,8 |
| 2018 | Pork | 79,0 | 101,1 |
| 2019 | Poultry | 25,6 | 31,3 |
| 2019 | Beef | 16,5 | 20,2 |
| 2019 | Pork | 66,5 | 81,2 |
| 2020 | Poultry | 25,1 | 32,6 |
| 2020 | Beef | 15,6 | 20,4 |
| 2020 | Pork | 68,8 | 89,6 |
| 2021 | Poultry | 22,0 | 27,8 |
| 2021 | Beef | 16,7 | 21,1 |
| 2021 | Pork | 61,0 | 77,0 |
Delivery quantities for pigs
The table shows the reported delivery quantities for pigs per active ingredient group in tons. A breakdown of the quantities dispensed for pigs by type of use is shown in the further table. This means, for example, that in 2021 a share of 29.8% of all dispensed antibiotics was used in pig fattening.
Dispensed quantities for the animal species pig per active ingredient group in tons for the years 2017 to 2021.
| Active ingredient group | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Tetracyclines | 15,72 | 18,38 | 13,43 | 14,64 | 12,69 |
| Extended-spectrum penicillins | 4,12 | 4,18 | 4,41 | 4,37 | 4,31 |
| Macrolides | 1,64 | 2,00 | 1,82 | 1,69 | 1,51 |
| Sulfonamides | 1,74 | 1,74 | 1,64 | 1,31 | 1,14 |
| Polymyxins | 0,77 | 0,78 | 0,87 | 1,03 | 0,87 |
| Aminoglycosides | 0,24 | 0,31 | 0,52 | 0,47 | 0,27 |
| Pleuromutiline | 0,16 | 0,25 | 0,27 | 0,27 | 0,23 |
| Trimethoprim and derivatives | 0,35 | 0,35 | 0,33 | 0,26 | 0,23 |
| Beta-lactamase sensitive penicillins | 0,20 | 0,19 | 0,20 | 0,21 | 0,21 |
| Fluoroquinolones | 0,10 | 0,10 | 0,10 | 0,11 | 0,11 |
| Lincosamide | 0,12 | 0,04 | 0,04 | 0,05 | 0,09 |
| other antibiotics | 0,15 | 0,07 | 0,05 | 0,05 | 0,08 |
| Amphenicols | 0,06 | 0,08 | 0,07 | 0,08 | 0,07 |
| 3rd +4th generation cephalosporins | 0,04 | 0,04 | 0,05 | 0,05 | 0,05 |
| Beta-lactamase resistant penicillins | <0,01 | <0,01 | <0,01 | <0,01 | <0,01 |
| 1st+2nd generation cephalosporins | <0,01 | <0,01 | <0,01 | <0,01 | <0,01 |
| Total | 25,41 | 28,53 | 23,81 | 24,58 | 21,86 |
Percentage of total sales volume for the animal species swine per type of use for the years 2017 to 2021.
| Type of use | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Other | 7,2% | 9,6% | 8,8% | 8,7% | 8,0% |
| Piglet rearing | 13,7% | 10,7% | 12,3% | 10,1% | 11,2% |
| Mast | 31,7% | 33,5% | 29,5% | 31,1% | 29,8% |
| Breeding | 21,2% | 22,5% | 21,3% | 23,5% | 21,5% |
| Total | 73,8% | 76,3% | 71,8% | 73,3% | 70,6% |
Delivery quantities for cattle
The table shows the reported release quantities for cattle per active ingredient group in metric tons, and the further table shows them as a percentage by type of use.
| Active ingredient group | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Tetracyclines | 3,88 | 3,76 | 3,28 | 3,36 | 3,80 |
| Beta-lactamase sensitive penicillins | 0,59 | 0,56 | 0,59 | 0,82 | 0,83 |
| Sulfonamides | 0,71 | 1,04 | 1,56 | 0,71 | 0,69 |
| Aminoglycosides | 0,25 | 0,27 | 0,38 | 0,49 | 0,47 |
| Penicillins with extended spectrum | 0,42 | 0,31 | 0,31 | 0,31 | 0,32 |
| Beta-lactamase resistant penicillins | 0,29 | 0,27 | 0,26 | 0,27 | 0,29 |
| Amphenicols | 0,16 | 0,13 | 0,17 | 0,15 | 0,15 |
| Trimethoprim and derivatives | 0,14 | 0,21 | 0,31 | 0,14 | 0,14 |
| Macrolides | 0,22 | 0,11 | 0,09 | 0,09 | 0,10 |
| 3rd +4th generation cephalosporins | 0,06 | 0,07 | 0,08 | 0,08 | 0,08 |
| Fluoroquinolones | 0,06 | 0,06 | 0,06 | 0,07 | 0,07 |
| 1st + 2nd generation cephalosporins | 0,03 | 0,03 | 0,02 | 0,03 | 0,04 |
| Other antibiotics | 0,03 | 0,02 | 0,04 | 0,03 | 0,02 |
| Lincosamide | 0,02 | 0,01 | 0,02 | 0,02 | 0,02 |
| Polymyxins | 0,02 | 0,02 | 0,01 | 0,01 | 0,01 |
| Pleuromutiline | <0,01 | <0,01 | <0,01 | <0,01 | <0,01 |
| Total | 6,89 | 6,86 | 7,19 | 6,59 | 7,02 |
Percentage of total sales volume for cattle per type of use for the years 2017 to 2021.
| Type of use | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Other | 2,5% | 2,5% | 2,7% | 3,0% | 3,9% |
| Mast | 6,9% | 6,0% | 7,1% | 7,0% | 7,7% |
| Fattened calf | 2,6% | 2,3% | 2,0% | 1,9% | 3,0% |
| Milk | 6,7% | 6,0% | 6,4% | 6,6% | 7,2% |
| Mother cow | 0,5% | 0,4% | 0,4% | 0,4% | 0,5% |
| Breeding | 0,8% | 1,2% | 3,2% | 0,7% | 0,3% |
| Total | 20,0% | 18,3% | 21,7% | 19,7% | 22,7% |
Quantities discharged for poultry
The following table shows the reported release quantities for poultry per active ingredient group in tons. Analogous to the previous chapters, the following table shows the quantities released in percent by type of use for poultry.
Release quantities for the animal species poultry per active ingredient group in tons for the years 2017 to 2021.
| Active ingredient group | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Extended-spectrum penicillins | 0,47 | 0,40 | 0,81 | 0,80 | 0,79 |
| Macrolides | 0,54 | 0,52 | 0,42 | 0,50 | 0,50 |
| Polymyxins | 0,46 | 0,50 | 0,27 | 0,31 | 0,28 |
| Tetracyclines | 0,23 | 0,14 | 0,27 | 0,32 | 0,15 |
| Sulfonamides | 0,19 | 0,24 | 0,19 | 0,14 | 0,13 |
| Fluoroquinolones | 0,06 | 0,07 | 0,05 | 0,07 | 0,05 |
| Lincosamide | <0,01 | 0,00 | 0,01 | 0,01 | 0,03 |
| Trimethoprim and derivatives | 0,04 | 0,05 | 0,04 | 0,03 | 0,03 |
| Aminoglycosides | 0,02 | 0,02 | 0,02 | 0,03 | 0,02 |
| Beta-lactamase sensitive penicillins | 0,01 | 0,01 | 0,01 | 0,01 | 0,01 |
| Amphenicols | 0,00 | 0,00 | <0,01 | 0,01 | 0,01 |
| Pleuromutiline | <0,01 | <0,01 | <0,01 | <0,01 | <0,01 |
| 3rd +4th generation cephalosporins | 0,00 | <0,01 | 0,00 | 0,00 | <0,01 |
| 1st + 2nd generation cephalosporins | 0,00 | <0,01 | 0,00 | 0,00 | 0,00 |
| Other antibiotics | <0,01 | 0,00 | <0,01 | 0,00 | 0,00 |
| Total | 2,04 | 1,95 | 2,10 | 2,23 | 1,99 |
Percentage of total sales volume for poultry by type of use for 2017 to 2021.
| Type of use | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Other | <0,1% | <0,1% | <0,1% | <0,1% | <0,1% |
| Parent animals | 0,4% | 0,2% | 0,5% | 0,7% | 0,5% |
| Pullets | 0,1% | 0,1% | 0,1% | 0,1% | 0,1% |
| Laying hens | 1,5% | 1,5% | 0,8% | 0,9% | 0,9% |
| Broiler chicken | 2,0% | 2,1% | 3,3% | 2,9% | 2,8% |
| Fattening turkey | 1,9% | 1,3% | 1,6% | 2,0% | 2,0% |
| Total | 5,9% | 5,2% | 6,3% | 6,7% | 6,4% |
Benchmarking reports
The term benchmarking generally stands for the comparison of a result with a reference value. As part of an international research project (Guidelines for Collection, Analysis and Reporting of Farm-level Antimicrobial Use, in the Scope of Antimicrobial Stewardship, www.aacting.org), a guideline was developed for antibiotic use, which covers the topics of data collection, data analysis, reporting and benchmarking. Benchmarking is seen as an effective tool for raising awareness and avoiding antibiotic use.
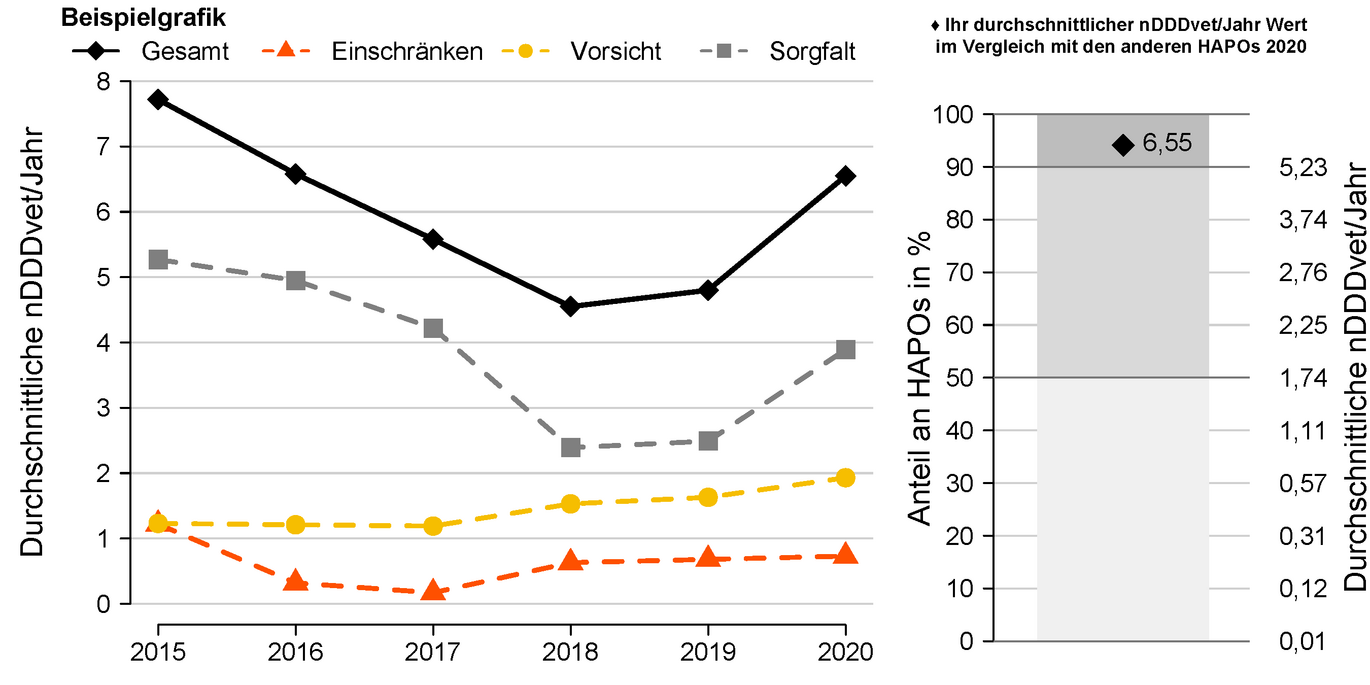
Taking this guideline into account, a benchmarking report was implemented for veterinarians managing home pharmacies. In addition to a temporal analysis of their own antibiotic dispensing quantities, this also includes a comparison with other veterinarians running their own pharmacies (benchmarking). As an indicator of antibiotic use, the quantities dispensed are converted into daily doses and standardized with the respective herd size (nDDvet/year). This results in the average number of days per year on which each animal on the farms in question was treated.
The evaluations are shown separately for the different types of animals and uses. Veterinarians managing in-house pharmacies can download their individual report via the portal https://eservices.basg.gv.at/, where they have to report their dispensing quantities.
In addition, benchmarking reports on their antibiotic consumption (based on dispensing quantities) are also prepared for pig farmers and made available via the Austrian Animal Health Services.
- Left part of the graph Temporal development of the values of nDDvet per year of an example HAPO separated according to the three summarized active ingredient categories and overall. The classification of active substances into categories has been redefined based on the recently published report of the EMA (European Medicines Agency, Committee for Medicinal Products for Veterinary use (CVMP), Committee for Medicinal Products for Human Use (CHMP) 2019).
- Right part of graph Comparison of total nDDvet per year with nDDvet per year of all other veterinary family pharmacies for the current evaluation year. The light gray area contains the HAPOs whose average nDDvet/year values are in the lower mean of all HAPOs (lower 50%). The dark gray area, on the other hand, contains the HAPOs whose average nDDvet/year values are in the upper 10%. The black check reflects the average nDDvet/year value of the example HAPO. If the dot is in the light gray area, this HAPO counts as one of the HAPOs with a low to medium average nDDvet/year value. If it is in the dark gray area, this HAPO counts among the 10% of HAPOs with the highest nDDvet/year values.
In the example shown, the average nDDDvet/year value is 6.55, placing this HAPO among the 10% of HAPOs with the highest nDDDvet/year values.
Discussion
At 39.07 metric tons, the distribution volume for 2021 shows the lowest value since data collection began (for the first time for 2010). The last few years have been characterized by greater fluctuations in distribution volumes, which can be explained in part by increased purchases for stock - due to uncertainty about the market situation and availability of medicines. The next data collection will show whether the reduction was sustainable.
Sales volumes of agents classified as Highest Priority Critically Important Antimicrobials (HPCIA) (WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance and World Health Organization 2017) have fluctuated between 4.64 and 5.78 tons over the past five years, and were 4.64 in 2021. Over the years, HPCIA have accounted for a relatively constant 12% to 13% of the total.
The mg/PCU indicator, which is a rough estimate of how many mg of antibiotics were sold per kg of live animal mass produced, decreased to 41.3 mg/PCU in 2021, 8.9% lower than in 2020. In absolute terms, this represents a decrease of 5 mg/PCU. The ratio of antibiotics purchased by HAPO in total to antibiotics dispensed in total was 76.8% in 2020 and 78.9% in 2021.
Based on the Austrian recording system, in which HAPOs must report their dispensing quantities per farm, animal species and type of use, it is possible to produce species-specific evaluations. The release quantities of the year 2021 show an increase for the animal species cattle (1.1 mg/PCU) and a decrease for the animal species pigs (-7.8 mg/PCU) and poultry (-3.1 mg/PCU). These values, as stated, reflect only a trend and are subject to some uncertainty.
In order to raise awareness and reduce antibiotic use, the project "Benchmarking report for veterinarians managing home pharmacies" was implemented in 2019 and the "Benchmarking report for pig-keeping farms" in the following year. In this project, veterinarians who manage their own pharmacies can download their individual report via the portal eservices.basg.gv.at and thus receive an indication of how they compare with their colleagues in terms of antibiotic dispensing. Pig farmers receive their reports through Austrian Animal Health Services, provided they give their consent. Work is also currently underway on a benchmarking system for cattle farmers.
- European Medicines Agency. 2011. "Trends in the Sales of Veterinary Antimicrobial Agents in Nine European Countries (2005-2009)." EMA/238630/2011.
- European Medicines Agency, Committee for Medicinal Products for Veterinary use (CVMP), Committee for Medicinal Products for Human Use (CHMP). 2019. "Categorisation of Antibiotics in the European Union." EMA/CVMP/CHMP/682198/2017.
- European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption. 2021. "Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020." EMA/58183/2021.
- R Core Team. 2022. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.
- WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance, and World Health Organization. 2017. Critically Important Antimicrobials for Human Medicine: Ranking of Antimicrobial Agents for Risk Management of Antimicrobial Resistance Due to Non-Human Use.
- World Health Organization Collaborating Centre for Drug Statistics Methodology. n.d. "WHOCC - ATCvet." ATCvet System for Classification of Veterinary Medicines. https://www.whocc.no/atcvet/
Last updated: 10.10.2023
automatically translated